Vaccine composition and applications thereof
A vaccine composition, Duck Riemer's technology, applied in the field of vaccine composition and its application, can solve the problems of short immunization period and inapplicability to duckling immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Preparation of Antigen
[0021] 1. Preparation of Serum Type Ⅰ Duck Hepatitis Virus Antigen
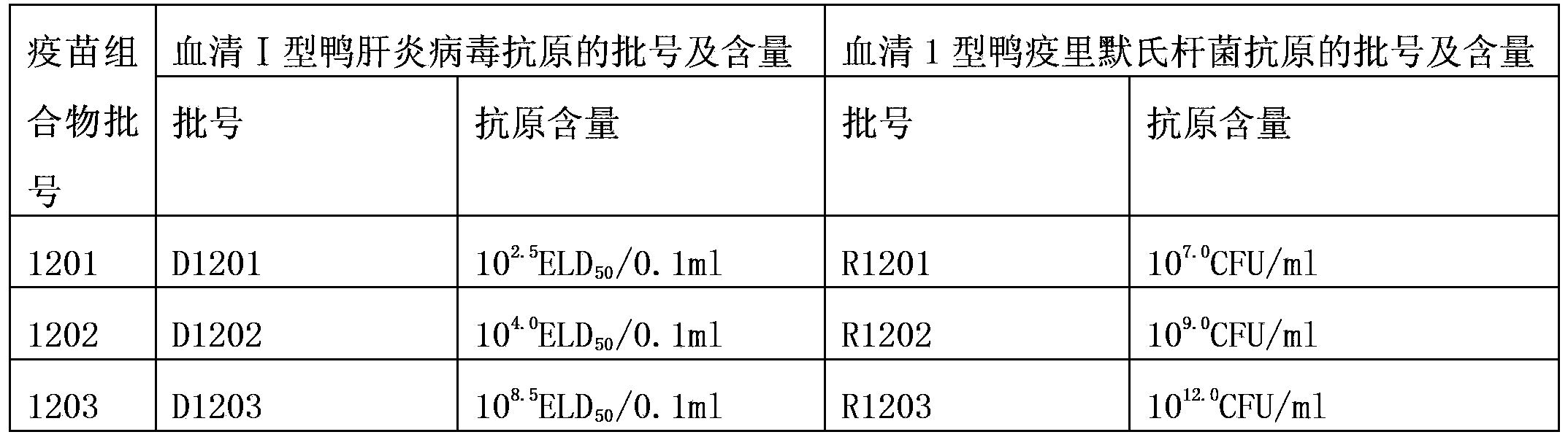
[0022] Take the attenuated duck hepatitis virus strain A66 of serum type Ⅰ, and use the published method (Zhang Xiaofei, Development of duck viral hepatitis attenuated vaccine (A66 strain) [D]. Nanjing: Nanjing Agricultural University, 2010), inoculate SPF chicken embryos Breeding was carried out, and the infected chick embryo fluid was harvested. The virus content of the infected chicken embryo fluid was determined to be 10 8.5 ELD 50 / 0.1ml, take part of the virus solution and dilute it to 10 4.0 ELD 50 / 0.1ml and 10 2.5 ELD 50 5% sucrose skim milk and vacuum freeze-dried to make serum type Ⅰ duck hepatitis virus antigens with different virus contents. The batch numbers are D1201, D1202 and D1203 respectively.
[0023] 2. Preparation of serotype 1 Riemerella anatipestifer antigen
[0024] Take the serotype 1 Riemerella anatipestifer Wuhan isolate, and use ...
Embodiment 2
[0025] Example 2. Preparation of vaccine
[0026] The prepared 3 batches of serum type 1 duck hepatitis virus antigen and 3 batches of serum type 1 Riemerella anatipestifer antigen were mixed separately as a vaccine composition (hereinafter referred to as "vaccine composition"), and 3 batches of vaccine combinations with different antigen contents were prepared. The batch numbers are 1201, 1202 and 1203 respectively, and each dose (0.3ml) of the mixed vaccine composition contains 1 dose (the content of the antigen is 10 2.5 ELD 50 / 0.1ml, 10 4.0 ELD 50 / 0.1ml and 10 8.5 ELD 50 / 0.1ml) serum type Ⅰ duck hepatitis virus antigen and 1 dosage (the content of the antigen before inactivation is 10 7.0 CFU / ml, 10 9.0 CFU / ml and 10 12.0 CFU / ml) of serotype 1 Riemerella anatipestifer antigen. The specific data of the vaccine composition is shown in Table 1.
[0027] The batch number and antigen content of the antigen used in the vaccine composition of table 13
[0028]
...
Embodiment 3
[0030] Application in embodiment 3. breeding ducks
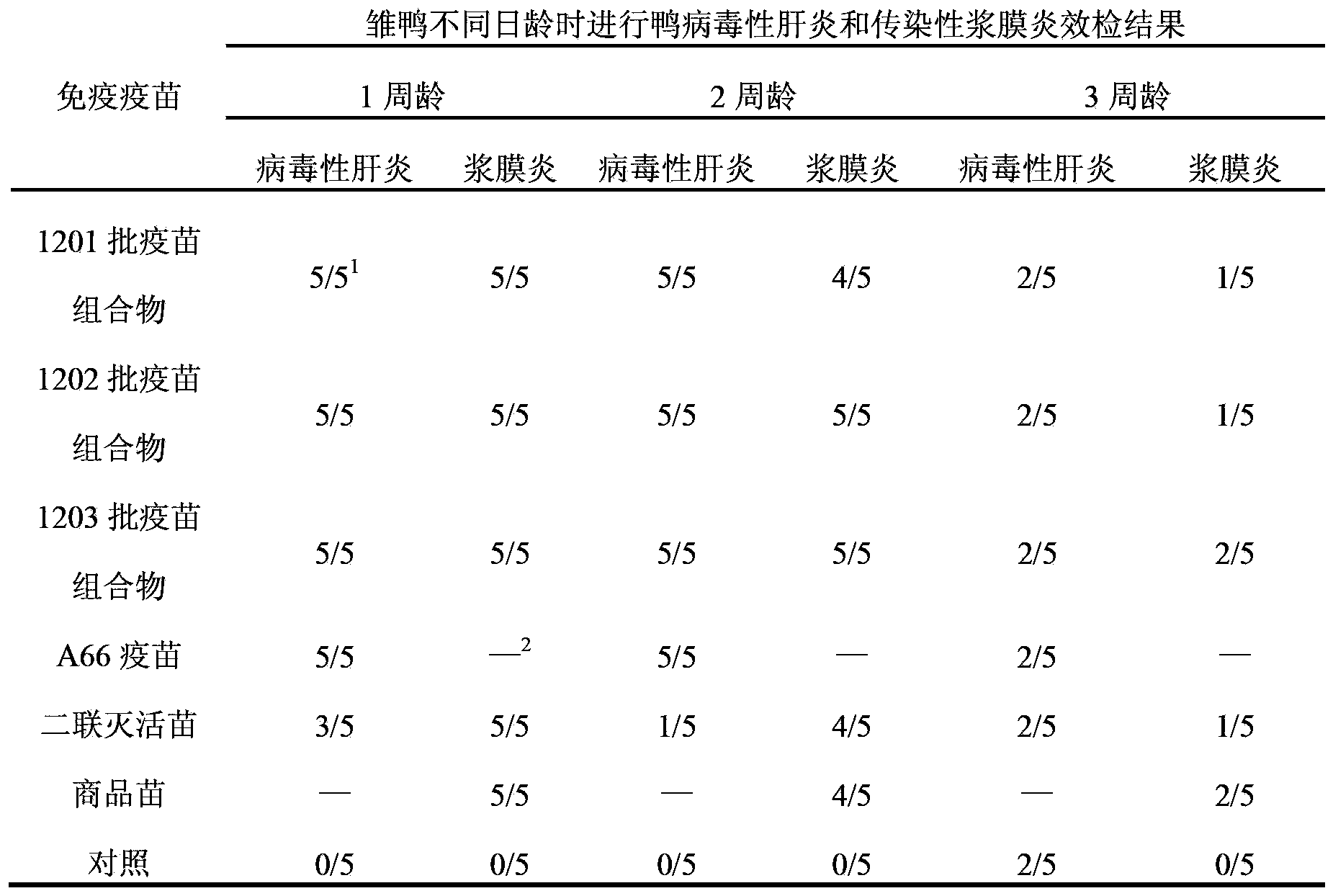
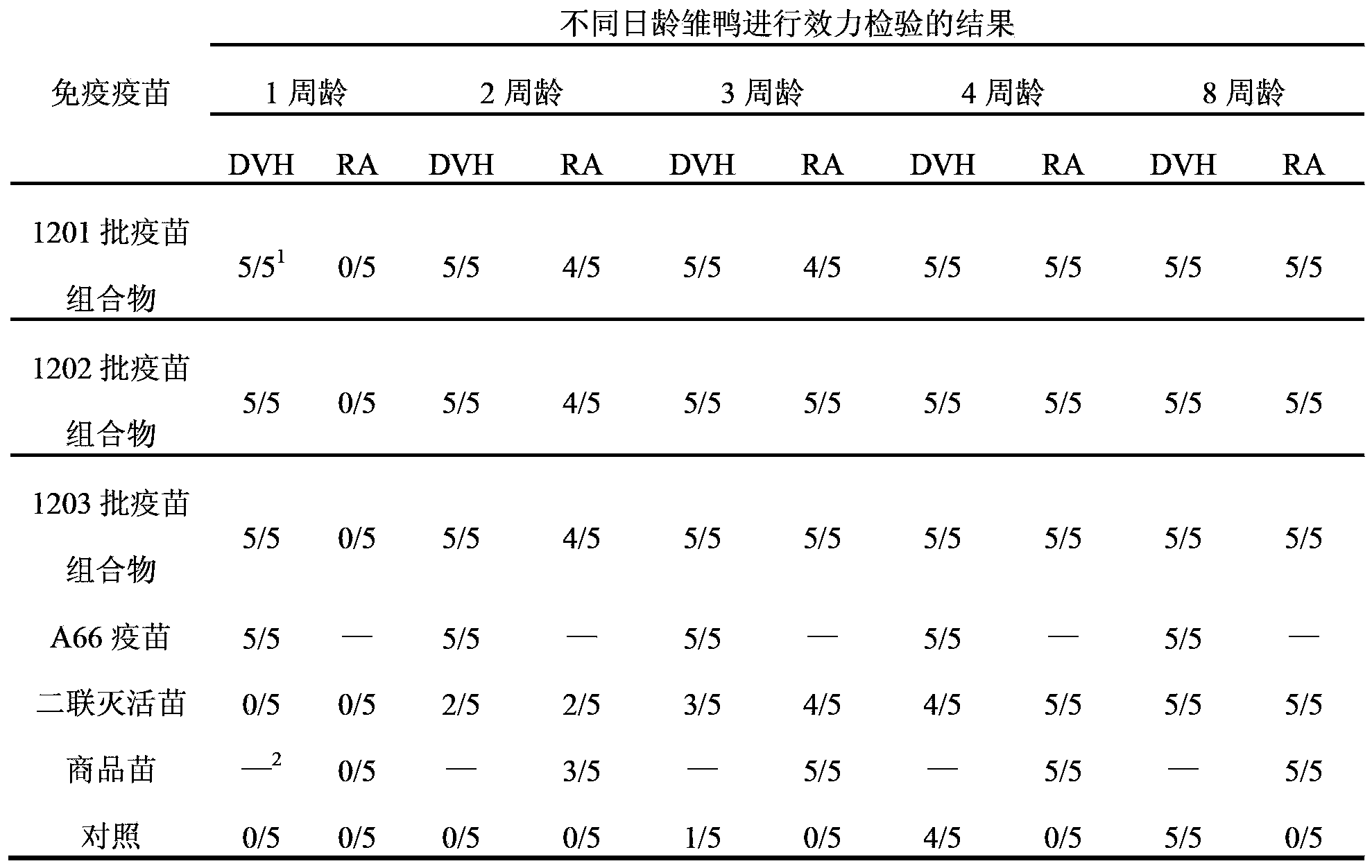
[0031]Take the 3 batches of vaccine compositions and control vaccines prepared in Example 2, and administer them once at the age of 110 days and 130 days in shelduck ducks (purchased from Luoyang Fumin Poultry Breeding Co., Ltd.) with one dose (0.3ml) respectively. Inject immunization, collect eggs hatched 20-30 days after the second injection, and hatch ducklings. When the ducklings were 1 week old, 2 weeks old and 3 weeks old, some ducklings were taken to test the efficacy of serum type 1 duck hepatitis virus and serum type 1 Riemerella anatipestifer. As a control, ducklings hatched from shelduck ducks without any immunization were used as control animals. The results are shown in Table 2.
[0032] The duration of passive immunity of ducklings hatched after table 2 adult female duck immunization
[0033]
[0034] Note: 1 The numerator represents the number of surviving ducks, and the denominator represents the tot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com