Pharmaceutical composition with effects of activating blood, removing stasis, reducing blood lipid and assisting in regulating cardiovascular and cerebrovascular diseases and preparation method thereof
A technology for cardiovascular and cerebrovascular diseases, promoting blood circulation and removing blood stasis, applied in cardiovascular system diseases, drug combinations, metabolic diseases, etc., can solve problems such as low absorption rate, unsuitable for hypotension patients, obvious toxic and side effects, etc., and achieve the goal of increasing bile acid Discharge, not easy to produce drug resistance, no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
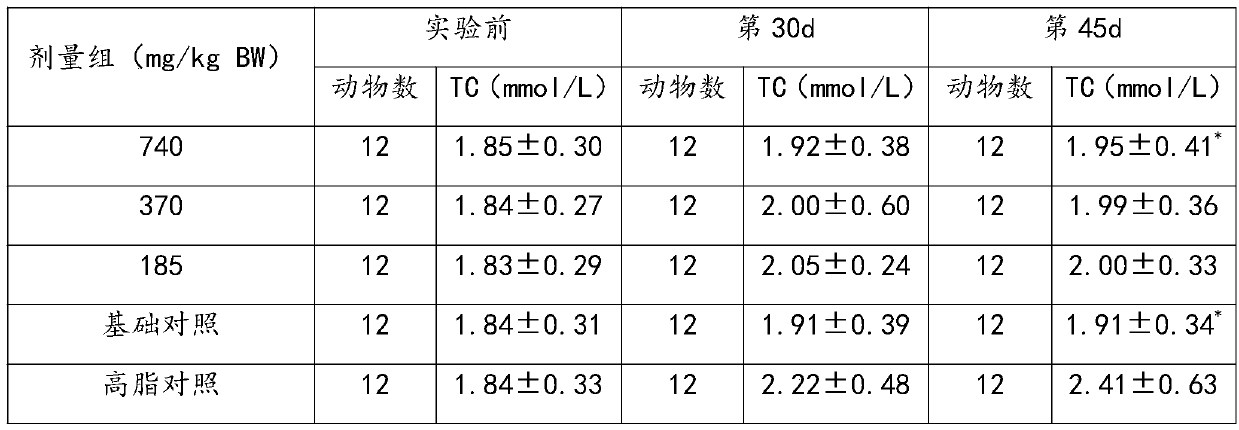
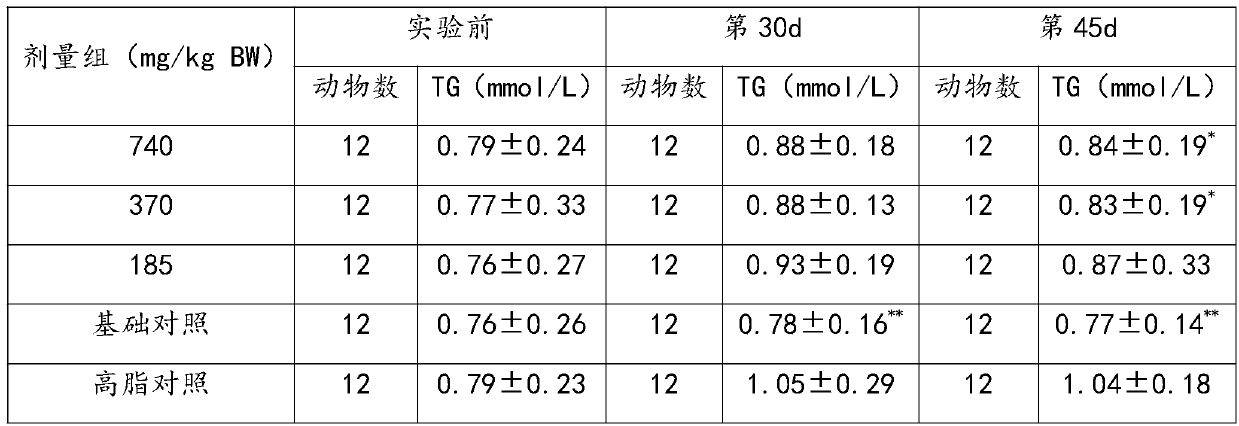
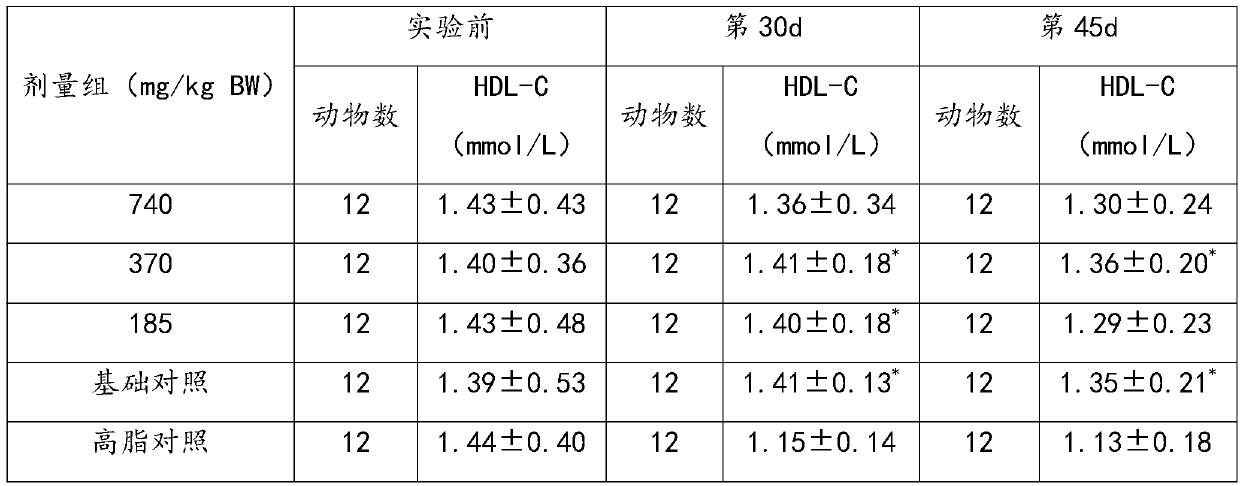
[0034] 1. A pharmaceutical composition for promoting blood circulation, removing blood stasis, lowering blood fat, and assisting in regulating cardiovascular and cerebrovascular diseases
[0035] The following raw materials are included in parts by weight: 30g of ginkgo leaf extract, 800g of kudzu root, 800g of salvia miltiorrhiza, and 800g of poria cocos.
[0036] 2. The preparation method of the pharmaceutical composition
[0037] Step 1. Select qualified kudzu root, salvia miltiorrhiza, and poria cocos, weigh them according to the proportion, wash and drain;
[0038] Step 2, adding the above materials into water for extraction and concentration to obtain a concentrated solution; the extraction conditions are: boiled twice, the first time is 10 times the amount of water, extracted for 2 hours; the second time is 8 times the amount of water, extracted for 2 hours ; The concentration is concentrated under reduced pressure, concentrated to a relative density of 1.1 to 1.2, a t...
Embodiment 2
[0043] 1. A pharmaceutical composition for promoting blood circulation, removing blood stasis, lowering blood fat, and assisting in regulating cardiovascular and cerebrovascular diseases
[0044] The following raw materials are included in parts by weight: 50 g of ginkgo leaf extract, 600 g of kudzu root, 600 g of salvia miltiorrhiza, and 600 g of poria cocos.
[0045] 2. The preparation method of the pharmaceutical composition
[0046] Step 1. Select qualified kudzu root, salvia miltiorrhiza, and poria cocos, weigh them according to the proportion, wash and drain;
[0047] Step 2, adding the above materials into water for extraction and concentration to obtain a concentrated solution; the extraction conditions are: boiled twice, the first time is 10 times the amount of water, extracted for 2 hours; the second time is 8 times the amount of water, extracted for 2 hours ; The concentration is concentrated under reduced pressure, concentrated to a relative density of 1.1 to 1.2,...
Embodiment 3
[0052] 1. A pharmaceutical composition for promoting blood circulation, removing blood stasis, lowering blood fat, and assisting in regulating cardiovascular and cerebrovascular diseases
[0053] The following raw materials are included in parts by weight: 90 g of ginkgo leaf extract, 1000 g of kudzu root, 1000 g of salvia miltiorrhiza, and 1000 g of poria cocos.
[0054] 2. The preparation method of the pharmaceutical composition
[0055] Step 1. Select qualified kudzu root, salvia miltiorrhiza, and poria cocos, weigh them according to the proportion, wash and drain;
[0056] Step 2, adding the above materials into water for extraction and concentration to obtain a concentrated solution; the extraction conditions are: boiled twice, the first time is 10 times the amount of water, extracted for 2 hours; the second time is 8 times the amount of water, extracted for 2 hours ; The concentration is concentrated under reduced pressure, concentrated to a relative density of 1.1 to 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com