A pharmaceutical composition, preparation and preparation method for promoting blood circulation, removing blood stasis and lowering blood fat
A technology for promoting blood circulation and removing blood stasis and composition, which is applied in the field of blood lipid-lowering pharmaceutical composition, promoting blood circulation and removing blood stasis, preparations and their preparations, which can solve the problems of obvious toxic and side effects, not treating the root cause of the index, and bad taste, etc., so as to reduce blood cholesterol Concentration, non-toxic and side effects, and the effects of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
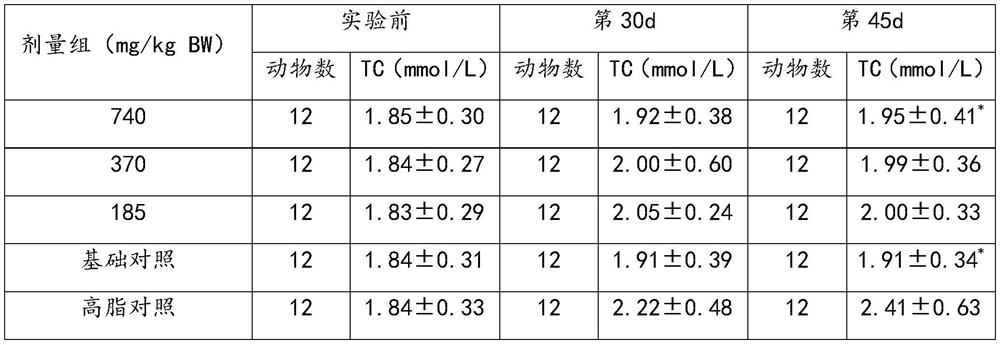
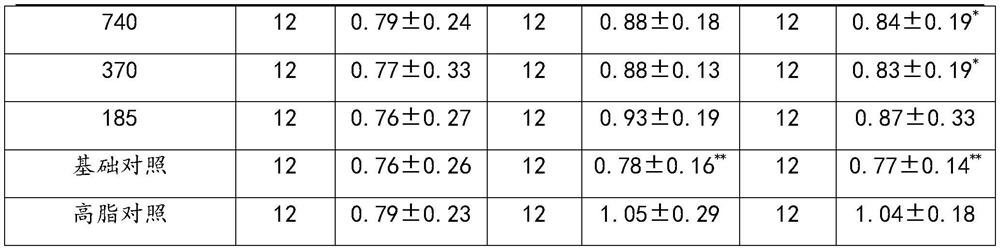
[0034] A stasis, hypolipidemic pharmaceutical composition
[0035] Comprising the following raw materials by weight: Ginkgo biloba extract 30g, Pueraria 800g, Salvia 800g, Poria 800g.
[0036] 2, preparation method of the pharmaceutical composition
[0037] Step 1, select qualified Pueraria, Salvia, Poria, according to the ratio that good weight, after washing with water and drain;
[0038] Step 2, the above material was added to water to extract and concentrated to give the concentrate; the extraction conditions: boiled twice, 10 times the first amount of water, extracting two hours; the second 8-fold amount of water, extracting two hours ; and concentrated under reduced pressure and the concentrate is concentrated to the relative density of 1.1 to 1.2, temperature is 70 ℃, the pressure is 0.06 ~ 0.09MPa;
[0039] Step 3, the solution was concentrated and dried to obtain a dry powder; drying conditions of the drying is spray-drying, the spray: inlet temperature 150 ~ 180 ℃, outle...
Embodiment 2
[0043] A stasis, hypolipidemic pharmaceutical composition
[0044] Comprising the following raw materials by weight: Ginkgo biloba extract 50g, Pueraria 600g, Salvia 600g, Poria 600g.
[0045] 2, preparation method of the pharmaceutical composition
[0046] Step 1, select qualified Pueraria, Salvia, Poria, according to the ratio that good weight, after washing with water and drain;
[0047] Step 2, the above material was added to water to extract and concentrated to give the concentrate; the extraction conditions: boiled twice, 10 times the first amount of water, extracting two hours; the second 8-fold amount of water, extracting two hours ; and concentrated under reduced pressure and the concentrate is concentrated to the relative density of 1.1 to 1.2, temperature is 70 ℃, the pressure is 0.06 ~ 0.09MPa;
[0048] Step 3, the solution was concentrated and dried to obtain a dry powder; drying conditions of the drying is spray-drying, the spray: inlet temperature 150 ~ 180 ℃, outle...
Embodiment 3
[0052] A stasis, hypolipidemic pharmaceutical composition
[0053] Comprising the following raw materials by weight: Ginkgo biloba extract 90g, Pueraria 1000g, Salvia 1000g, Poria 1000g.
[0054] 2, preparation method of the pharmaceutical composition
[0055] Step 1, select qualified Pueraria, Salvia, Poria, according to the ratio that good weight, after washing with water and drain;
[0056] Step 2, the above material was added to water to extract and concentrated to give the concentrate; the extraction conditions: boiled twice, 10 times the first amount of water, extracting two hours; the second 8-fold amount of water, extracting two hours ; and concentrated under reduced pressure and the concentrate is concentrated to the relative density of 1.1 to 1.2, temperature is 70 ℃, the pressure is 0.06 ~ 0.09MPa;
[0057] Step 3, the solution was concentrated and dried to obtain a dry powder; drying conditions of the drying is spray-drying, the spray: inlet temperature 150 ~ 180 ℃, ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com