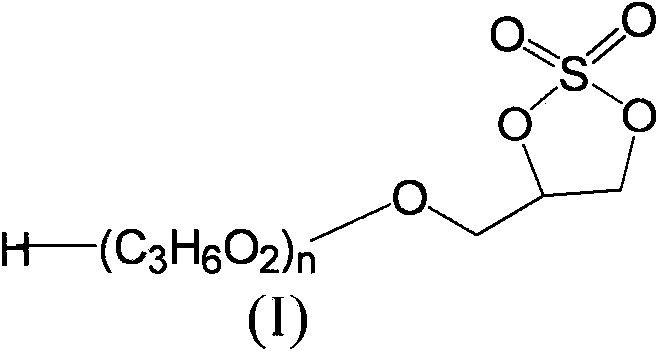
Cyclic (poly)glycerol sulphates and preparation and use thereof
A kind of glycerol sulfate, cyclic technology, applied in the intermediate field of surfactant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0080] Example 1: Method for preparing the compound of formula (I')
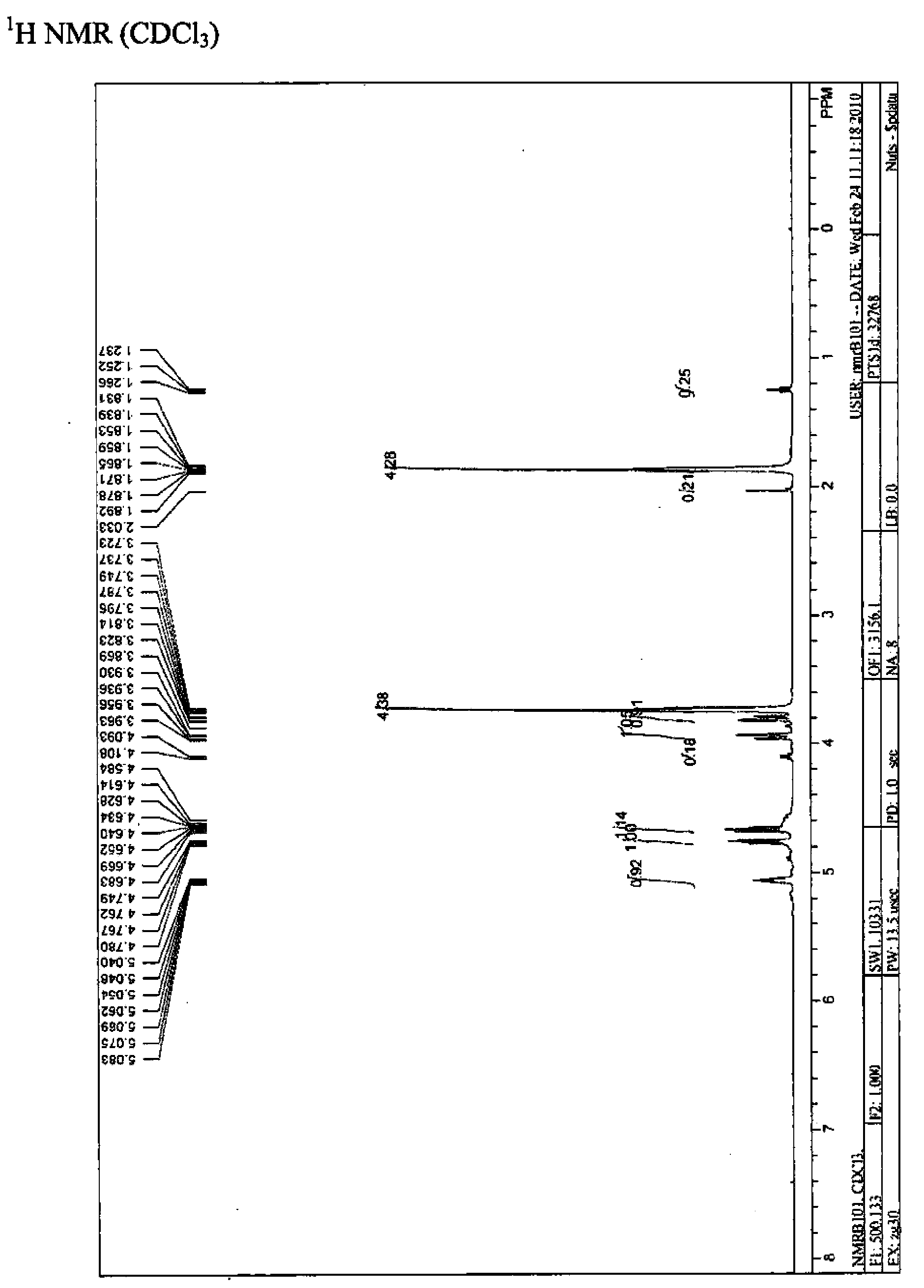
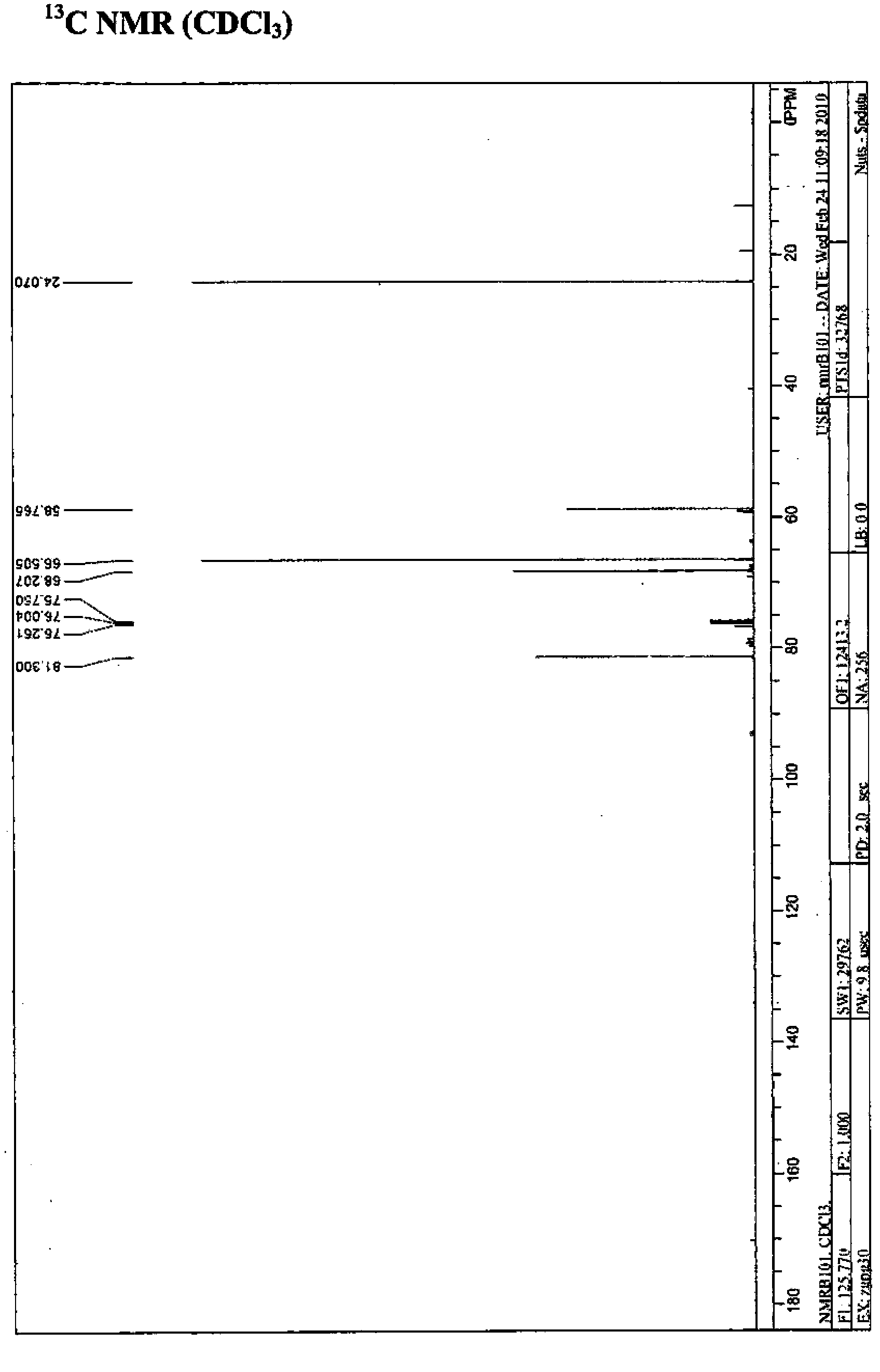
[0081] Add 6.9g of cyclic sulfite (4-(hydroxymethyl)-1,3,2-dioxapentane-2-oxide (50mmol)) and 50mL of acetonitrile to a 250mL three-necked round bottom flask , 100mgRuCl 3 .3H 2 O (0.75mmol) and 16g NaIO 4 (75 mmol), the mixture was cooled to 0-5°C by an ice-salt bath. Then 75 mL of cold water was added to the mixture and the temperature was increased to 30°C. The mixture was stirred for 5 minutes, and the mixture became a green suspension. Combine 400mL ethyl acetate and 40mL saturated NaHCO 3 The aqueous solution is added to the mixture. The mixture was separated into two phases. The aqueous phase was extracted with ethyl acetate (100ml×2). The combined organic phase was washed with 40 mL of water and subjected to anhydrous Na 2 SO 4 dry. The solvent was removed by a rotary evaporator to produce 5.6 g of the desired yellow liquid product, crude yield: 72.7%.
[0082] 1 H NMR(CDCl 3 , 500MHz), δ: 5.05-5....
example 2
[0084] Example 2: Method for preparing cyclic diglycerol sulfate
[0085]
[0086] Add sulfite II (50mmol), 50mL acetonitrile, 100mg RuCl as shown above into a 250mL three-neck round bottom flask 3 .3H 2 O (0.75mmol) and 16g NaIO 4 (75 mmol), the mixture was cooled to 0-5°C by an ice-salt bath. Then 75 mL of cold water was added to the mixture and the temperature was increased to 30°C. The mixture was stirred for 5 mins, and the mixture became a green suspension. Combine 400mL ethyl acetate and 40mL saturated NaHCO 3 The aqueous solution is added to the mixture. The mixture was separated into two phases. The aqueous phase was extracted with ethyl acetate (100ml×2). The combined organic phase was washed with 40 mL of water and subjected to anhydrous Na 2 SO 4 dry. The solvent was removed by a rotary evaporator to produce 6.0 g of the desired yellow liquid product, crude yield: 52.7%.
example 3
[0087] Example 3: Application example
[0088] 7.64g of N,N-dimethyldodecane-1-amine and 25ml of THF were added to a 50ml three-necked round bottom flask, 5.36g of CGS (a compound of formula (I')) (in 10ml of THF) was added dropwise in about 10 minutes, and then heated to reflux using an oil bath and stirred for a period of time. Many bubbles were observed at the bottom of the flask. Then, the reaction mixture was cooled to room temperature and filtered, washed with THF and dried. 7.9 g of the following sulfate betaine was obtained as a white solid.
[0089] HOCH 2 CH(OSO 3 - )CHN + (CH 3 ) 2 (CH 2 ) 11 CH 3
[0090] The final product of the reaction 1 H NMR(DMSO-d 6 )(CDCl 3 , 500MHz), δ: 5.1 (m, 1H); 4.5 (m, 1H); 3.75 (m, 1H); 3.3-3.51 (m, 4H); 3.1 (d, 6H); 1.61-1.69 (m, 2H) ); 1.29 (m, 18H); 0.84 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com