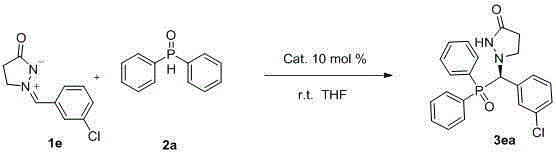
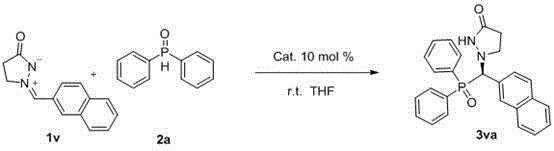
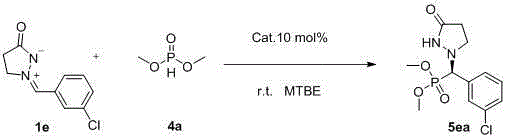A kind of synthetic method of chiral phosphorus-containing pyrazolone compound
A synthetic method and technology of pyrazolone, which is applied in the field of catalytic synthesis of chiral pyrazolidinone aminophosphorus compounds, can solve the problem that the process yield is not very high, there is no universal adaptability, and the substrate application range is not wide and other problems, to achieve the effect of less dosage, excellent corresponding selectivity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Add dihydroquinine thiourea (5.9mg, 0.01mmol), 2a (20.2mg, 0.1mmol), 1a (17.4mg, 0.1mmol) sequentially into the reaction flask, add 0.5mL tetrahydrofuran, and react at room temperature for 4 hours , the reaction system removes the solvent, and the crude product can be obtained by simple column chromatography (eluent: ethyl acetate:dichloromethane=3:2) to obtain the target product 3aa (35.3mg), white solid, yield 94%, mp224-226°C, 95%ee.
[0036] Add dihydroquinine square amide (1.5mg, 0.0025mmol), and 2a (20.2mg, 0.1mmol), 1a (17.4mg, 0.1mmol) in turn into the reaction flask, add 0.5mL tetrahydrofuran, and react at room temperature for 5 hours , the reaction system removes the solvent, and the crude product can be obtained by simple column chromatography (eluent: ethyl acetate:dichloromethane=3:2) to obtain the target product 3aa (20.6mg), white solid, 55% yield, mp224-226°C, 67%ee.
[0037] Add dihydroquinine square amide (3.3mg, 0.005mmol), and 2a (20.2m...
Embodiment 2
[0041]
[0042] Dihydroquinine squaramide (6.5mg, 0.01mmol) successively in the reaction vial, and 2a (20.2 mg, 0.1 mmol), 1b (19.2mg, 0.1mmol), add 0.5mL tetrahydrofuran, react at room temperature for 30 minutes, remove the solvent from the reaction system, and the crude product is subjected to simple column chromatography (eluent is ethyl acetate:dichloromethane=3:2 ) to get the target product 3ba (37.8 mg), white solid, yield 96%, mp 208-210°C.
[0043] The product was analyzed and the results were as follows: >99%ee, determined by HPLC [DaicelChiralcelOD-H, hexanes / i -PrOH=70 / 30, flow rate: 1.0mL min –1 ,λ=254.4nm,t(major)=7.850,t(minor)=10.599];[α] D 25 =–41.28(c0.625, CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ8.47(s,1H),7.81(dt, J =18.2,7.6Hz,3H),7.54–7.39(m,5H),7.29(td, J =7.4,1.1Hz,1H),7.25–7.19(m,2H),7.14(dd, J =13.9,6.7Hz,1H),700–6.85(m,2H),5.02(d, J =10.3Hz,1H),3.51(dd, J =19.9,9.0Hz,1H),3.09(td, J =10.0,6.6Hz,1H),2.00–2.00(m,1H),1.78–1.66(m,1H); 13 CNM...
Embodiment 3
[0045]
[0046] Dihydroquinine squaramide (6.5mg, 0.01mmol) successively in the reaction vial, and 2a (20.2 mg, 0.1 mmol), 1c (19.2mg, 0.1mmol), add 0.5mL tetrahydrofuran, react at room temperature for 30 minutes, remove the solvent from the reaction system, and the crude product is subjected to simple column chromatography (eluent is ethyl acetate:dichloromethane=3:2 ) to get the target product 3ca (37.4 mg), white solid, yield 95%, mp 201-203°C.
[0047] The product is analyzed and the results are as follows: 99%ee, determined by HPLC [DaicelChiralcelAD-H, hexanes / i -PrOH=70 / 30, flow rate: 1.0mL min –1 ,λ=210.8nm,t(major)=7.657,t(minor)=15.051;[α] D 25 =–28.00(c0.525,CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ8.72(s,1H),7.80–7.71(m,2H),7.53–7.37(m,5H),7.36–7.27(m,3H),7.26–7.18(m,2H),6.84(t, J =8.5Hz,2H),4.45(d, J =9.4Hz,1H),3.39(dd, J =19.7,9.1Hz,1H),3.23–3.07(m,1H),2.02–1.91(m,1H),1.82–1.67(m,1H); 13 CNMR (101MHz, CDCl 3 )δ173.51,163.05(d, J =250.7Hz), 132.99(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com