Nanoparticle tumour vaccines
A nanoparticle, vaccine technology, applied in the field of nanoparticle-mediated delivery, which can solve the problems of discomfort and human use, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1 - Synthesis and Characterization of Nanoparticles
[0143] The test ligands and their identification numbers are given below (molecule wt);
[0144] SIINFEKL (963) (SEQ ID NO: 87)
[0145] SIINFEKL-N-(CH 2 ) 2 -SH (1021)
[0146] FLSIINFEKL-N-(CH 2 ) 2 -SH (1280) (SEQ ID NO: 88)
[0147] FLAAYSIINFEKL-N-(CH 2 ) 2 -SH (1587) (SEQ ID NO: 89)
[0148] AAYSIINFEKL-N-(CH 2 ) 2 -SH (1325) (SEQ ID NO:90)
[0149] HS(CH 2 ) 2 -CONH-SIINFEKL (1051)
[0150] HS(CH 2 ) 2 -CONH-FLSIINFEKL (1309)
[0151] HS(CH 2 ) 2 -CONH-FLAAYSIINFEKL (1616)
[0152] HS(CH 2 ) 2 -CONH-AAYSIINFEKL (1356)
[0153] HS-(CH 2 ) 10 -(CH 2 OCH 2 ) 7 -CONH-SIINFEKL (1471)
[0154] HS-(CH 2 ) 10 -(CH 2 OCH 2 ) 7 -CONH-FLSIINFEKL (1732)
[0155] HS-(CH 2 ) 10 -(CH 2 OCH 2 ) 7 -CONH-FLAAYSIINFEKL (2034)
[0156] HS-(CH 2 ) 10 -(CH 2 OCH 2 ) 7 -CONH-AAYSIINFEKL (1774)
[0157] Test NPs were synthesized using 10 μmole of gold chloride (Aldrich484385), 30...
Embodiment 2
[0193] Example 2 - Evaluation of Presentation Assays
[0194] The T cell receptor (TCR) is on the surface of T lymphocytes and recognizes peptides in the context of the major histocompatibility complex (MHC) (1). In general, antigen-presenting cells (APCs) contain machinery to process proteins and load them onto empty MHC. While CD4+ T cells recognize MHC class II (MHC II), CD8+ T cells respond to MHC class I (MHC I). Previously, MHCII peptides were derived from endocytic components of the extracellular milieu. In contrast, MHCI is loaded with processed peptides from cellular endogenous sources (1,2).
[0195] SIINFEKL (SEQ ID NO: 87), is a peptide epitope from ovalbumin (OVA) in a protein called H-2K b are presented in the context of the murine MHCI allele (3). If OVA is expressing H-2K b SIINFEKL (SEQ ID NO: 87) is routinely presented in mouse cells. However, if OVA is provided exogenously, SIINFEKL can be presented by an alternative method called MHCI cross-presentati...
Embodiment 3
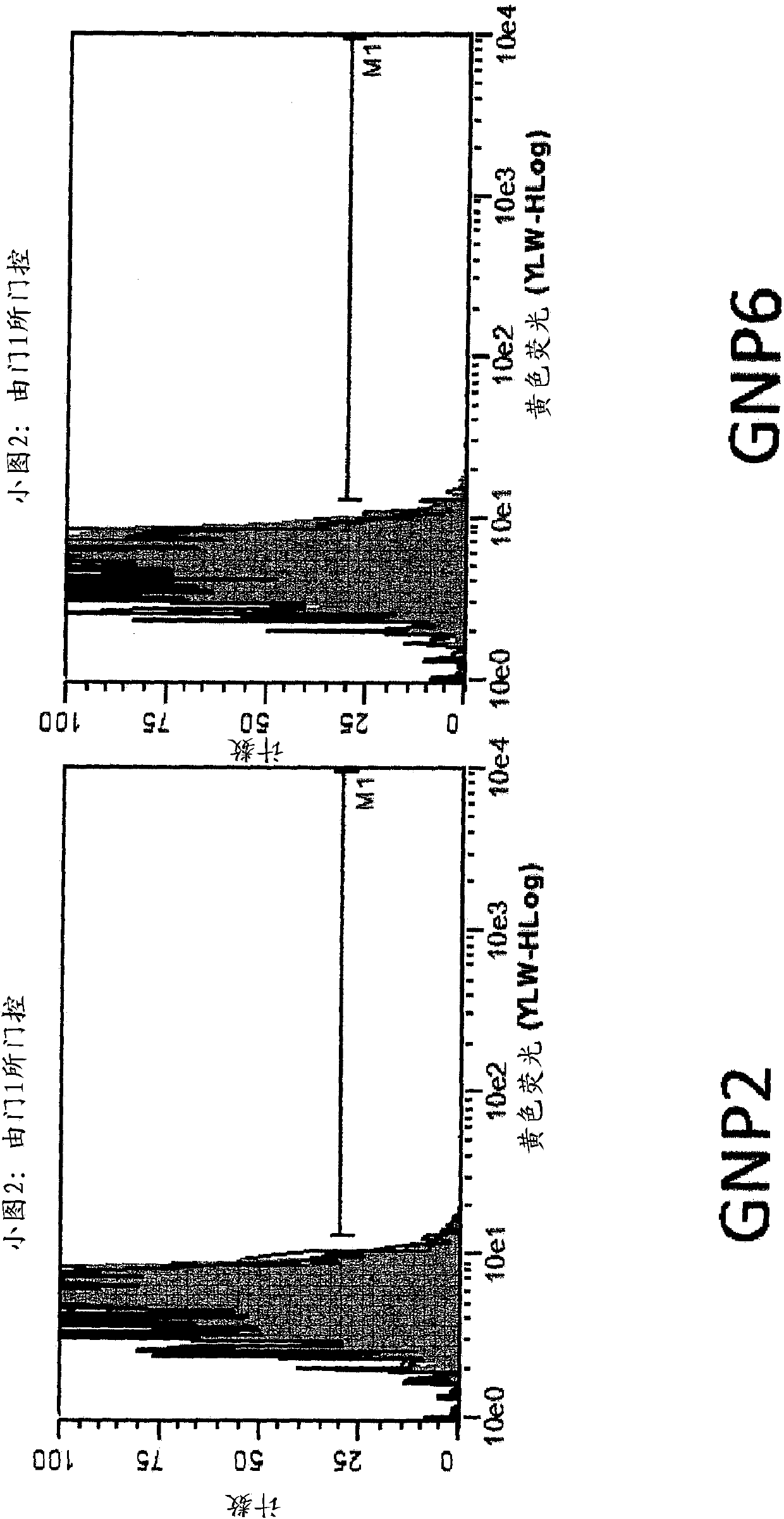
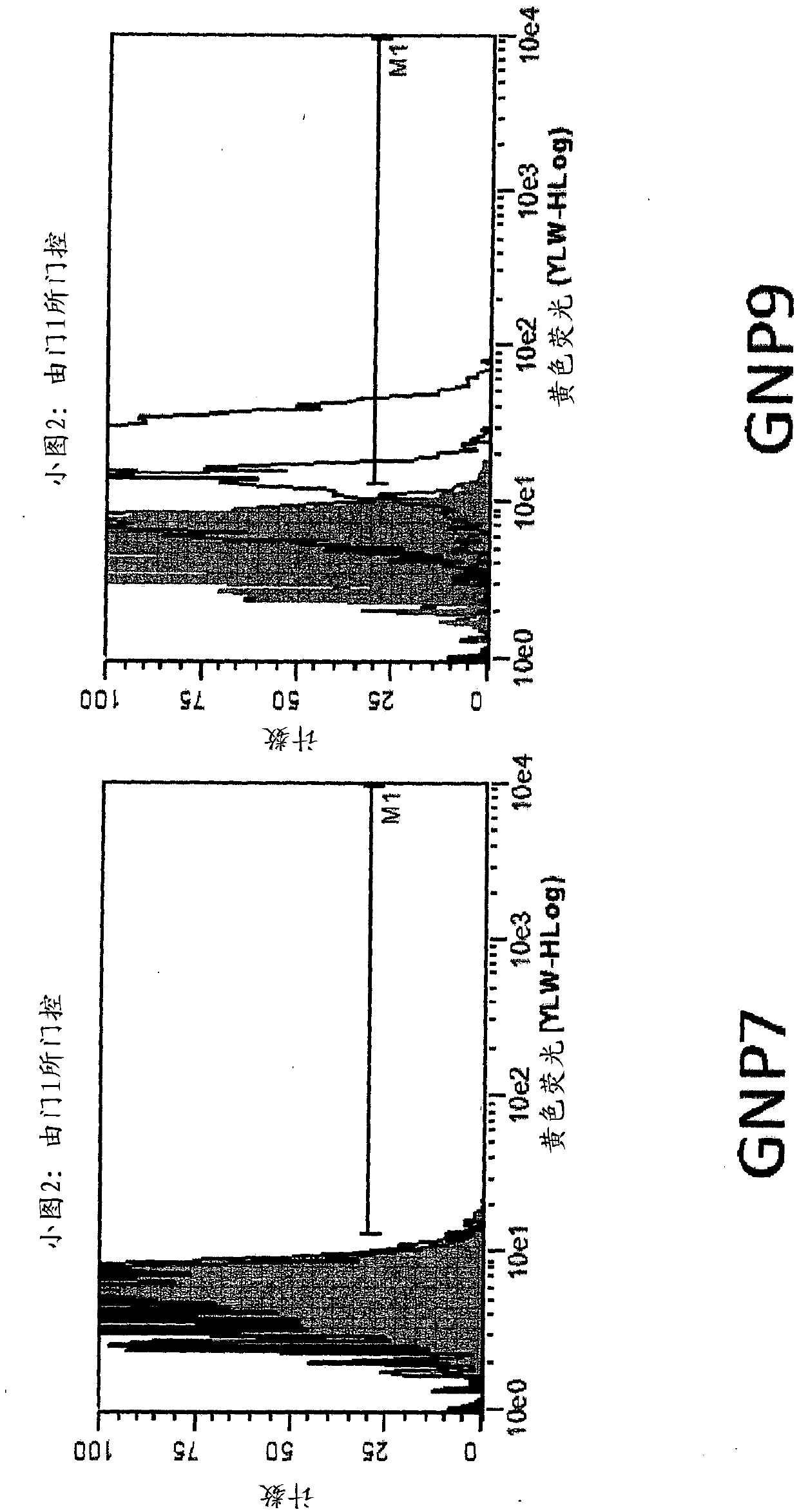
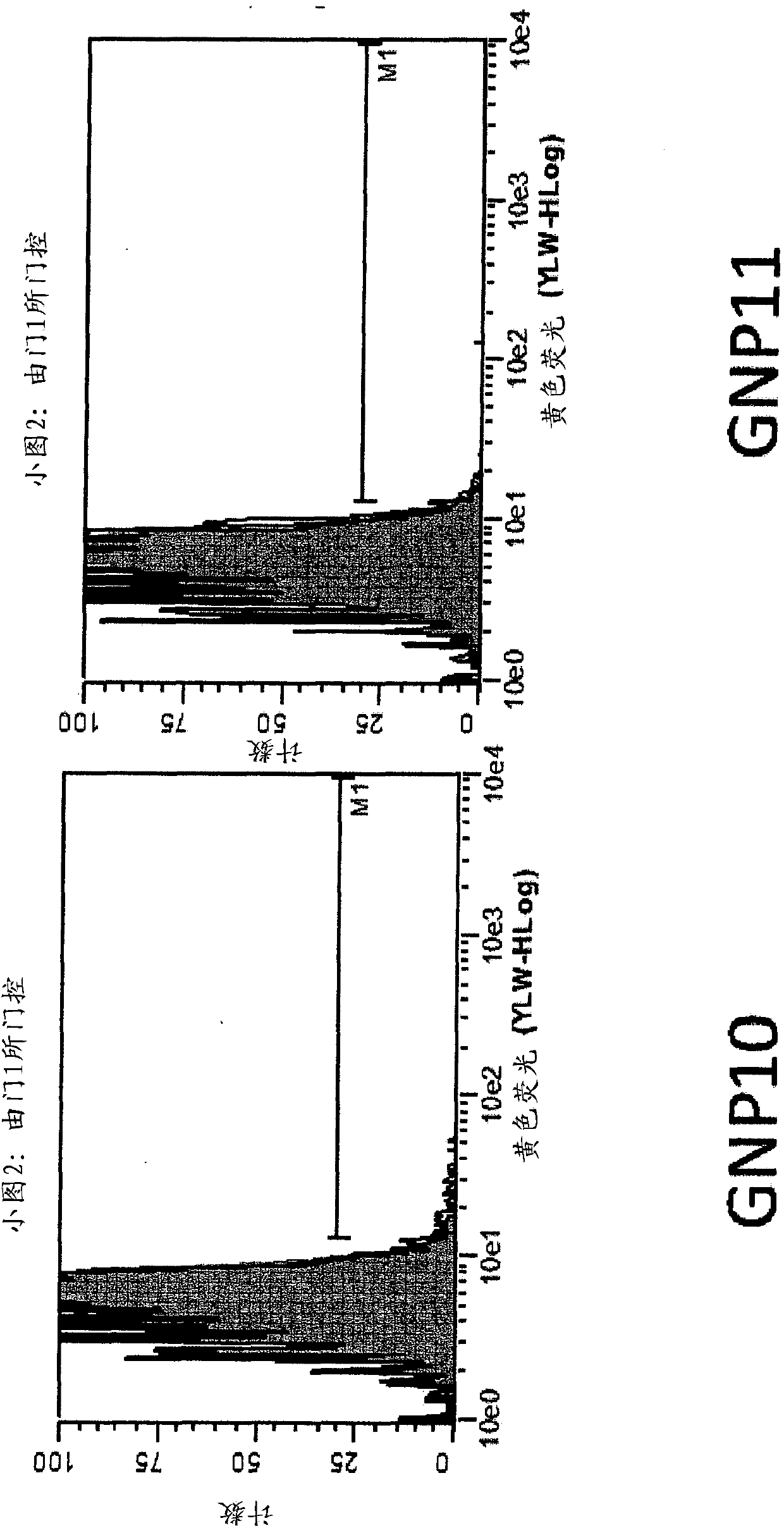
[0223] Example 3 - Nanoparticle-peptide presentation assay
[0224] The test ligands listed below were constructed and chemically attached to gold nanoparticles (GNPs) via the linkers described above.
[0225] 1. SIINFEKL (SEQ ID NO: 87)
[0226] 2. SIINFEKL-N-(CH2)2-SH
[0227] 3. FLSIINFEKL-N-(CH2)2-SH (SEQ ID NO: 88)
[0228] 4. FLAAYSIINFEKL-N-(CH2)2-SH (SEQ ID NO: 89)
[0229] 5. AAYSIINFEKL-N-(CH2)2-SH (SEQ ID NO:90)
[0230] 6. HS(CH2)2-CONH-SIINFEKL
[0231] 7. HS(CH2)2-CONH-FLSIINFEKL
[0232] 8. HS(CH2)2-CONH-FLAAYSIINFEKL
[0233] 9. HS(CH2)2-CONH-AAYSIINFEKL
[0234] 10.HS-(CH2)10-(CH2OCH2)7-CONH-SIINFEKL
[0235] 11.HS-(CH2)10-(CH2OCH2)7-CONH-FLSIINFEKL
[0236] 12.HS-(CH2)10-(CH2OCH2)7-CONH-FLAAYSIINFEKL
[0237] 13.HS-(CH2)10-(CH2OCH2)7-CONH-AAYSIINFEKL
[0238] SIINFEKL (SEQ ID NO: 87) is an epitope from ovalbumin that is presented in the context of the murine MHC I molecule H-2Kb and measured using two methods. One approach utilizes a TCR-like anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com