Cationic displacer molecules for hydrophobic displacement chromatography
A technique of hydrophobic cation, displacement chromatography, applied in the field of cation displacer molecules for hydrophobic displacement chromatography, which can solve the problem that the displacer compound does not work well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6b
[0196] (for Example 6b(a))
[0197] (2) Select the initial concentration of the sample by one of two methods:
[0198] (a) Initial sample concentration (mg / mL) = 0.25x replacement agent concentration (mM) x formula weight (mg / μmole)
[0199] = 0.12 x 10 mM x 1.7466 mg / μmole = 2.10 mg / mL (for Example 6b(a))
[0200] (b) Select an estimated column binding capacity for the sample, eg 50 mg sample / mL matrix. Assuming the displacement flow rate and the sample loading flow rate are the same:
[0201] Initial sample concentration (mg / mL) = (column binding capacity (mg / mL) m ) x column volume (mL m ) / ((T 2 -T 1 ) x sample flow rate (mL / min))
[0202] =(50mg / mL m x4.155mL m ) / ((586min-270min)x0.208mL / min)
[0203] =3.16mg / mL
[0204] (for Example 6b(a))
[0205] If the first DC experiment with loaded samples resulted in an overloaded condition (>100% loading), the experiment was rerun at half the sample concentration. From the results of the first successful DC experiment...
Embodiment 1a
[0233] Example 1a: Exemplary protocol. Displacement chromatography purification of crude synthetic angiotensin I
[0234] Instrument configuration : Single main pump with 4 solvent lines, sample injection valve with 40mL loop, column bypass valve
[0235] Sample injection valve: 6-port valve controlled by single-channel toggle logic (S3=0, bypass loop, S3=1, loop inline)
[0236] Column Bypass Valve: 6-way valve controlled by single channel switching logic (S6=0, column inline, S6=1, bypass column)
[0237] A post-column UV photodiode array detector (flow cell: 0.5 mm flow, 10 μL volume) followed by a conductivity detector (flow cell: 170 μL volume). Bypass the conductivity cell when collecting fractions for analysis.
[0238] Loading buffer = A-buffer (S1 = 1, flow on, S1 = 0 flow off); Displacer buffer = B-buffer (S2 = 1, flow on, S2 = 0 flow off);
[0239] Displacer removal buffer = C-buffer (S4 = 1, flow on, S4 = 0 flow off); column storage buffer = D-buffer (S5 = 1,...
Embodiment 1b
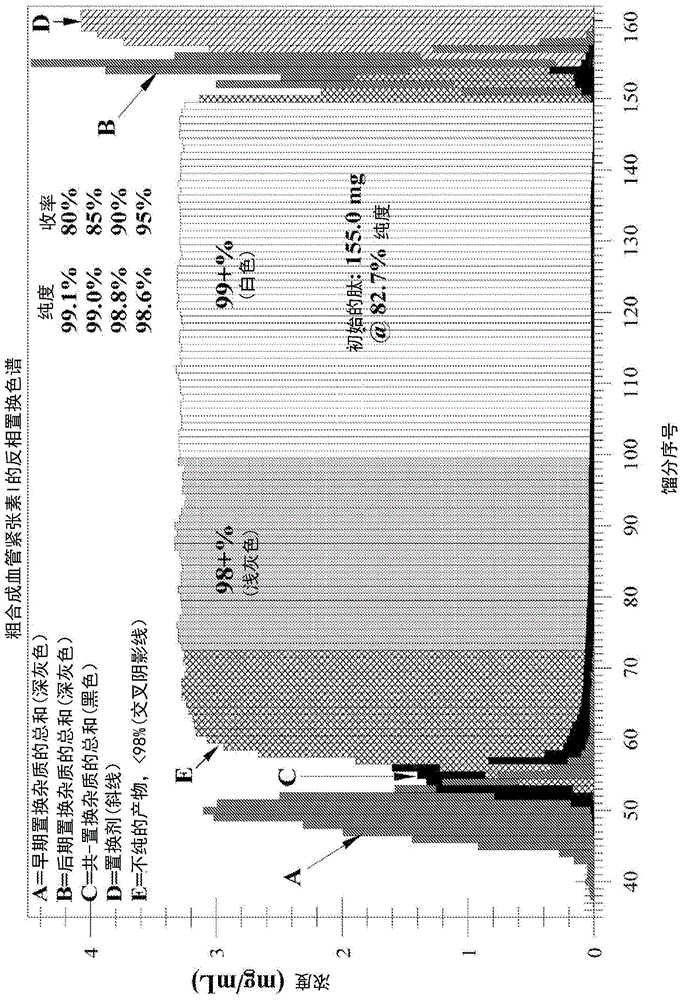
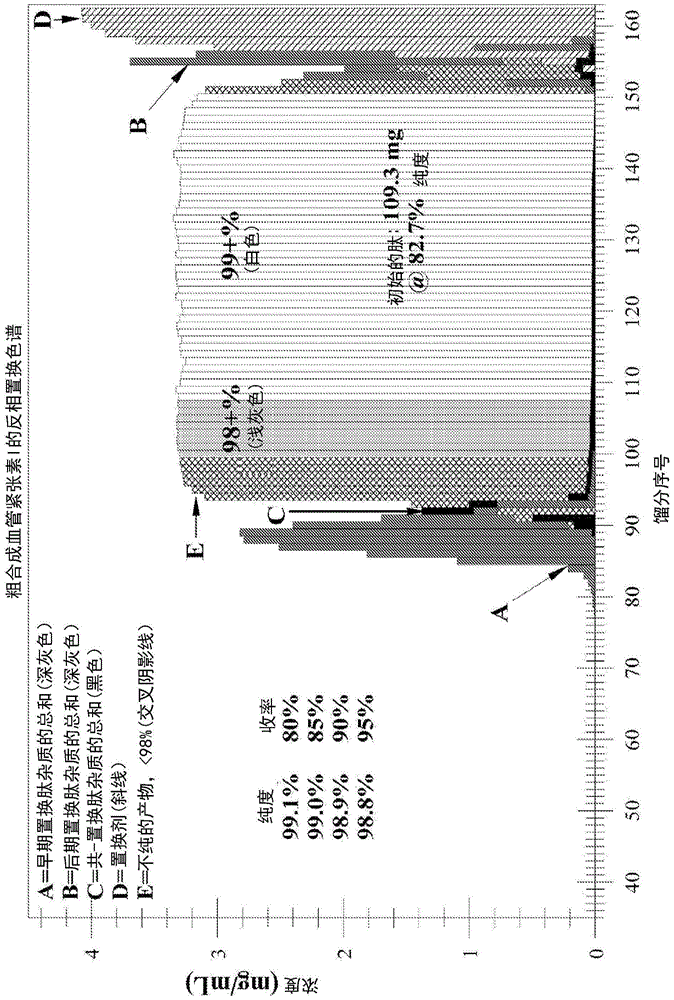
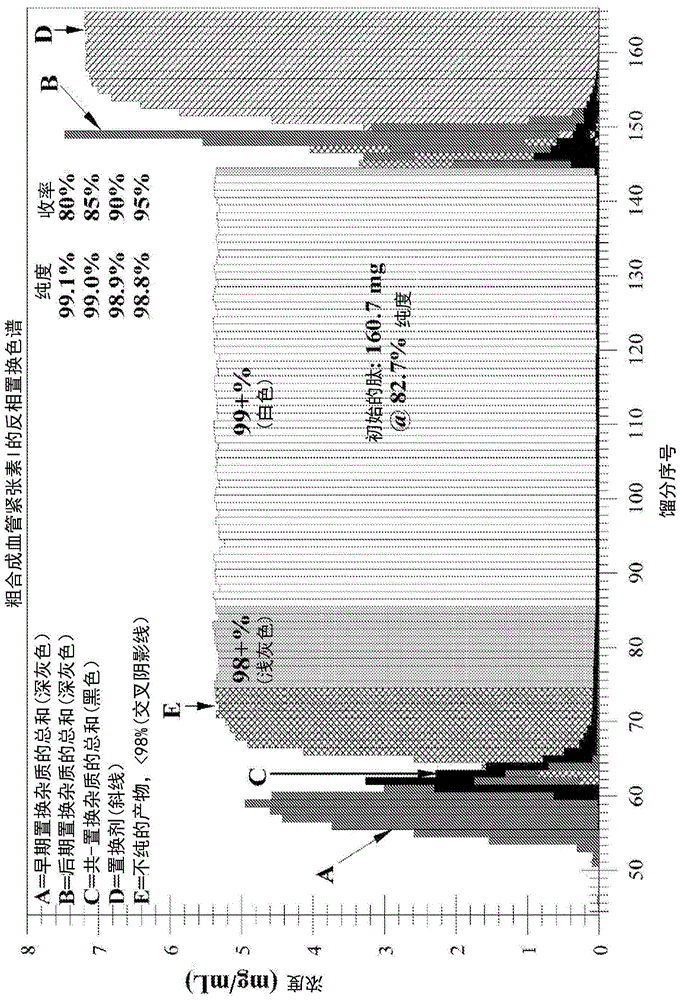
[0246] Example 1b: Displacement Chromatographic Purification of Crude Angiotensin I Using Displacer 14 - Higher Loading at Lower Concentrations (see Figure 1b -analyze)
[0247] Operating conditions:
[0248] Starting peptide: desalted crude synthetic angiotensin I, 82.7% pure, FW ~ 1.296 mg / μmole, charge = +4
[0249] Column: Waters Xbridge BEH130, 5 μm, 4.6x250mm SS, -C on silicone 18
[0250] Flow Rate: Load = 208 μL / min; Displacement = 208 μL / min
[0251] Ion-pairing reagent: trifluoroacetate (CF 3 CO 2 - )
[0252] Temperature: 23°C
[0253] pH: 2.0
[0254] Displacer Buffer: 10.0 mM Displacer 14 + 12 mM CF 3 CO 2 H contains 3% (v / v) MeCN in DI water, pH = 2.0 with NH 4 Oh
[0255] Loading buffer: 12 mM TFA with 3% (v / v) MeCN in water, pH=2.0 with NH 4 Oh
[0256] Sample solution: 4.38mg / mL peptide in water containing 3% (v / v) MeCN and 27mM CF 3 CO 2 - ;pH=2.0 contains NH 4 Oh
[0257] Loading: 155.0mg, 35.4mL, from 40mL loop;
[0258] Loading ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com