Vaccine composition for mucosal administration
A vaccine composition and mucosal administration technology, applied in the directions of vaccines, drug combinations, drug delivery, etc., can solve the problems of giving difficult medical workers acupuncture accident patients, heat-labile enterotoxin, etc., and avoid acupuncture infection. Risk of accident, improvement of quality of life, effect of improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
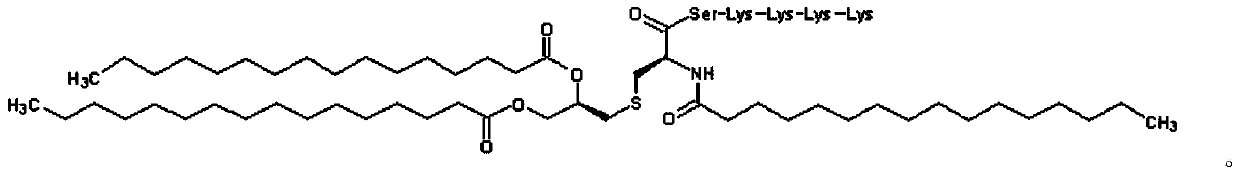
[0149] Liquid formulations administered sublingually
[0150] Liquid formulations having the compositions shown in Tables 1 to 9 below were produced and used as administration samples for mouse immunization tests. Specifically, 20 parts by weight of an additive (DMSO) and physiological saline as a base were added to the compounding amounts of antigenic peptides and cellular immunity induction promoters described in Tables 1 to 9, and a desired pharmacologically acceptable acid, The total amount was 100 parts by weight, and mixed to prepare a liquid preparation for sublingual administration.
[0151] GPC3 peptide, survivin 2B peptide, HER2 / neu_A24 peptide, MAGE3_A24 peptide, IPEP87 peptide, HER2 / neu E75 peptide, PR1 peptide, HER2 / neu_A02 peptide, MAGE3_A02 peptide, HBVenv peptide, and Peptide-25 were chemically synthesized using HPLC Refined products. OVA protein was purchased from Sigma-Aldrich.
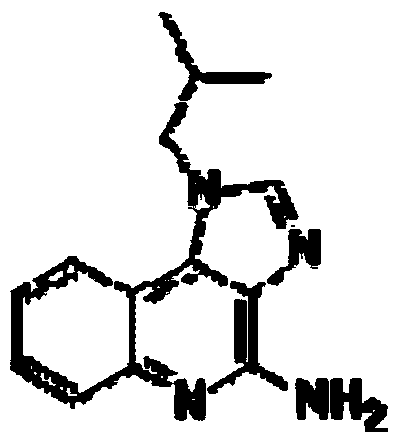
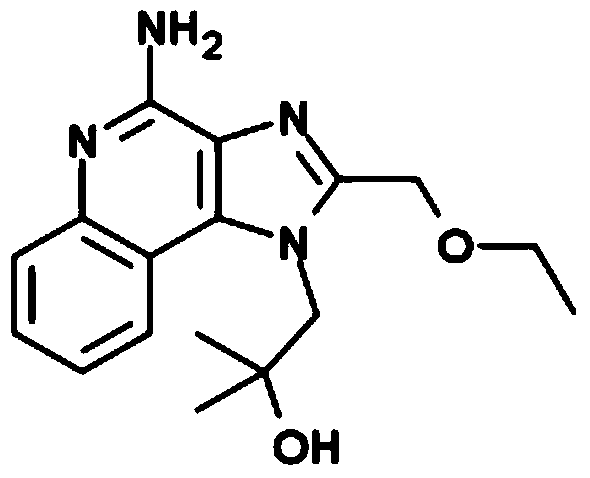
[0152] Imiquimod was purchased from Tokyo Chemical Industry. Cyclic di-GMP (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com