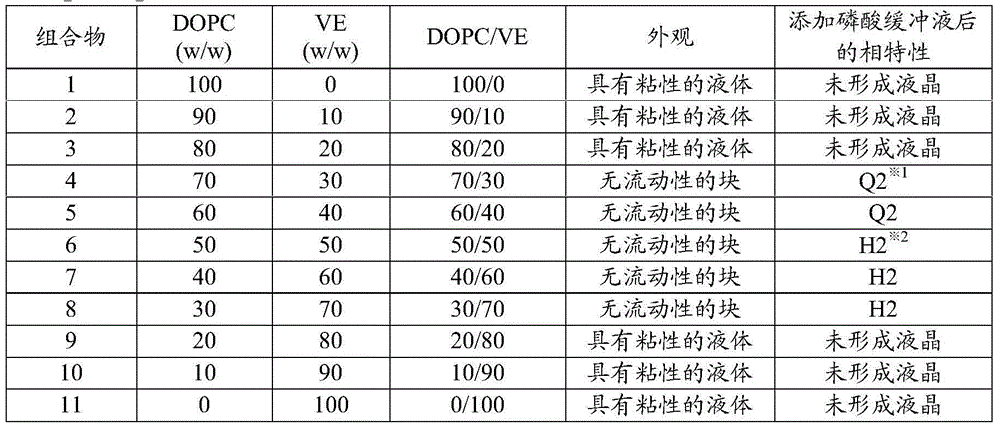
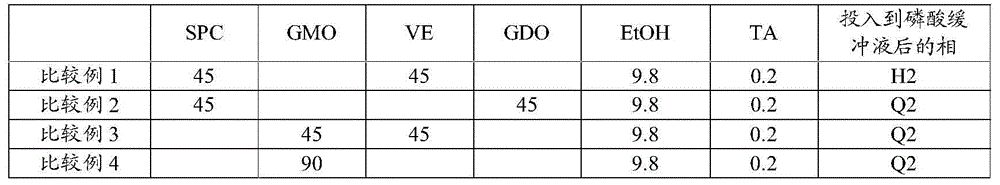
Non-aqueous liquid composition
A liquid composition, non-aqueous technology, applied in the fields of injections and ophthalmic preparations, can solve problems such as insufficient drug sustained release, and achieve excellent drug sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 2
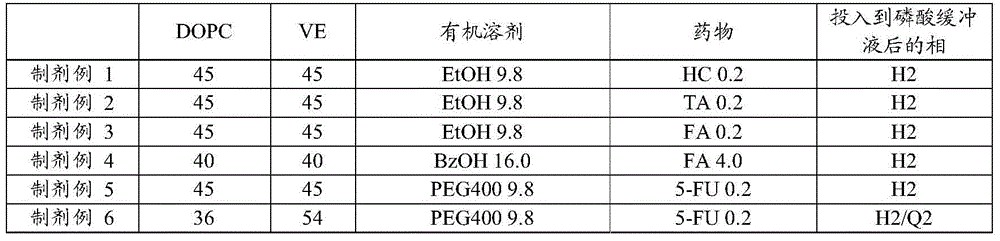
[0047] A DOPC formulation containing 0.2% of TA was prepared in the same manner as in Formulation Example 1 except that 20 mg of fludroxycortisol acetate (TA) was used instead of HC.
preparation example 3
[0049] A DOPC formulation containing 0.2% of FA was prepared in the same manner as in Formulation Example 1 except that 20 mg of fluocinolone acetate (FA) was used instead of HC.
[0050] (4) Preparation Example 4
[0051] Add about 200 mg of benzyl alcohol (BzOH) to 50 mg of FA, heat to 60° C. and stir until dissolved (20% FA solution). This 100 mg of 20% FA solution was added to 200 mg of DOPC, and dissolved while heating to 70° C. while stirring. After dissolving, 200 mg of VE was added, heated to 70° C. and stirred until dissolved to prepare a DOPC formulation containing 4% FA.
[0052] (5) Preparation Example 5
[0053] Add 6 mg of fluorouracil (5-FU) to 294 mg of polyethylene glycol (PEG) 400, heat to 70°C and stir until dissolved (2% 5-FU solution). This 50 mg of 2% 5-FU solution was added to 225 mg of DOPC, and dissolved while heating to 70° C. while stirring. After dissolving, 225 mg of VE was added, heated to 70° C. and stirred until dissolved, and a DOPC prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com