ANTIBODIES TO CD1d
An antibody and antigen technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Example 1-Generation of anti-CD1d antibodies
[0221] Phage display
[0222] FAbs that bind to CD1d / β2M in humans and cynomolgus monkeys were isolated from the phagemid library used for the first experiment.
[0223] Anti-CD1d / β2MFAbs were isolated from the phage display library during the two panning "activities" (i.e., isolated phage display tests with different reagents or panning conditions). The general test procedure follows the method outlined by Marks et al. (Marks, J.D. & Bradbury, A., 2004, Methods Mol Biol, 248, 161-66).
[0224] Each phage display event includes three rounds of panning. For each round, by mixing 1:1 with blocking buffer (5% skim milk in phosphate buffered saline, pH 7.4) and incubating for 1 hour at room temperature, the ~1×10 13 The phage particles are blocked. The blocked phage library was then incubated with 100 microliters of streptavidin-conjugated Dynabead (Invitrogen) for 45 minutes to pre-exclude the streptavidin binding agent, and the str...
Embodiment 2
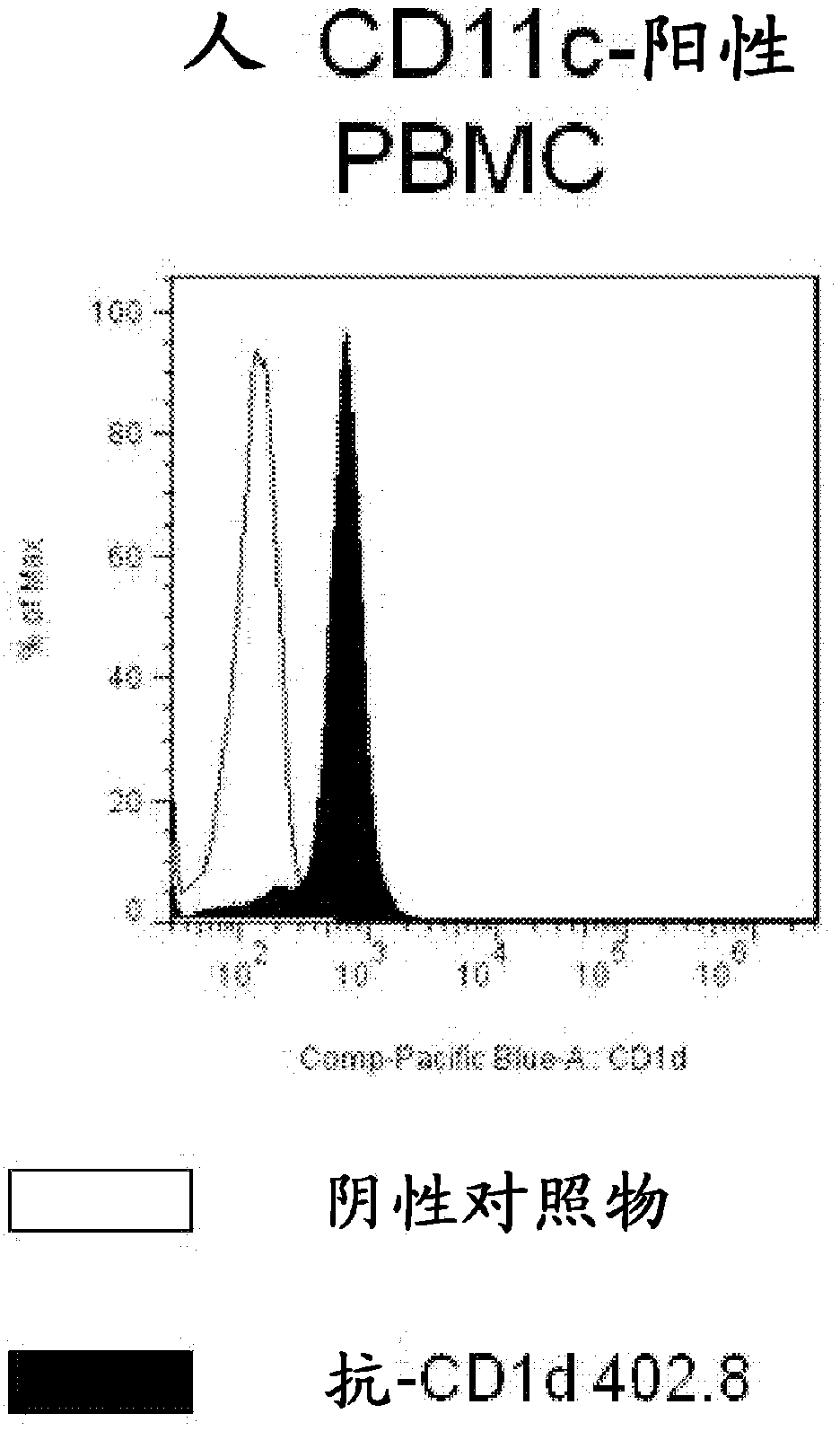
[0237] Example 2-Confirmation of IgG binding to CD1d
[0238] The human-cynomolgus CD1d-reactive FAb was converted into IgG4 format, expressed and purified as described in the general method. The purified antibodies were tested for binding to human and cyno CD1d by ELISA and SPR using the modified version of the assay described in Example 1. In short, for the ELISA assay, a Maxisorp ELISA plate (Nunc) was coated at 1 μg / ml with the appropriate antigen. The plate was then washed and the purified IgG sample was added to the individual wells of the ELISA plate. The IgG was allowed to bind to the captured CD1d / β2M at room temperature for one hour, and then washed three times with PBS-T and three times with PBS. The bound IgG was detected using HRP-conjugated antibody against human Fc (Sigma). The detection antibody was incubated for 30 minutes at room temperature. The plate was washed to remove unbound antibody, and the assay signal was visualized by incubating with 50 microliter...
Embodiment 3
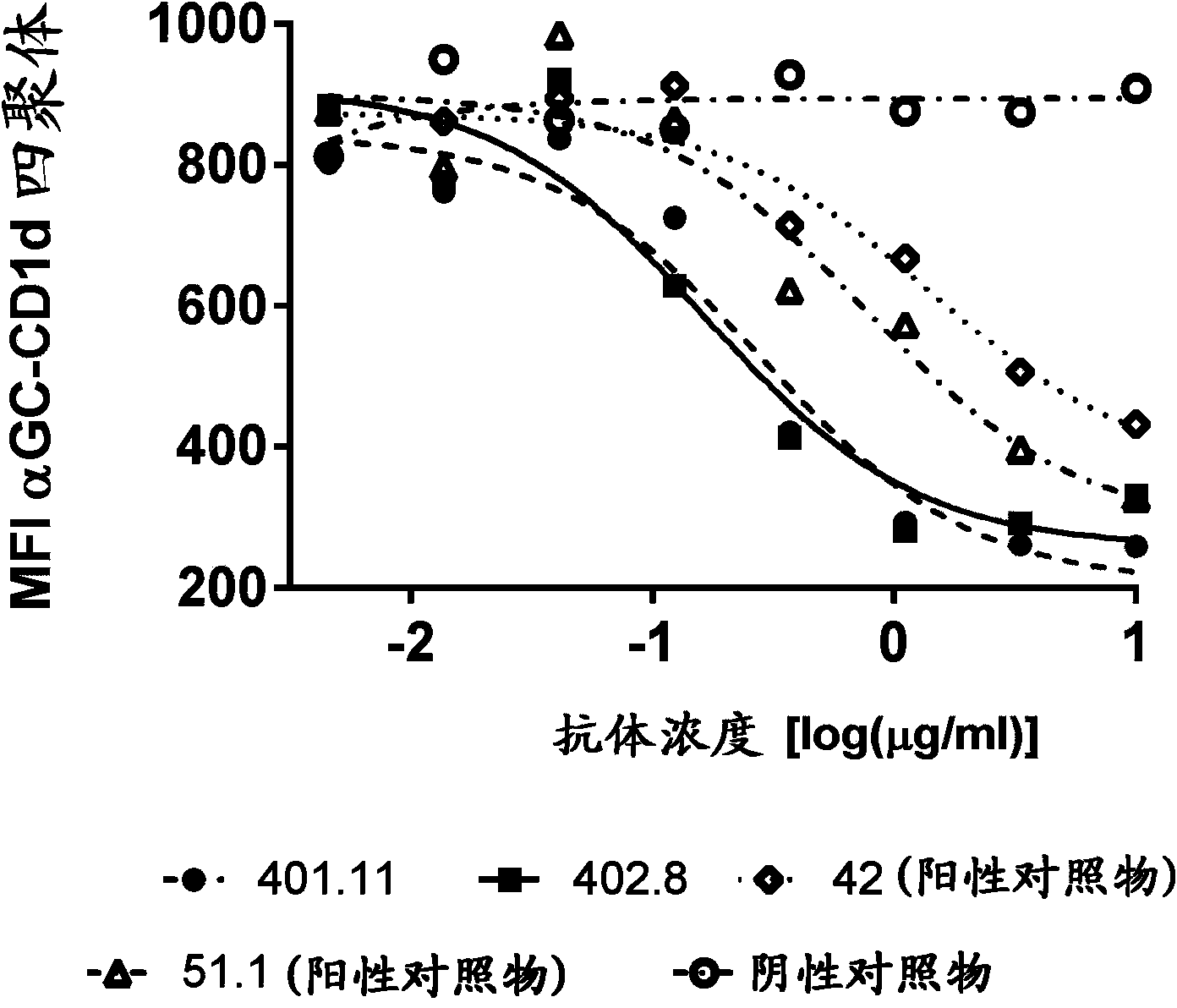
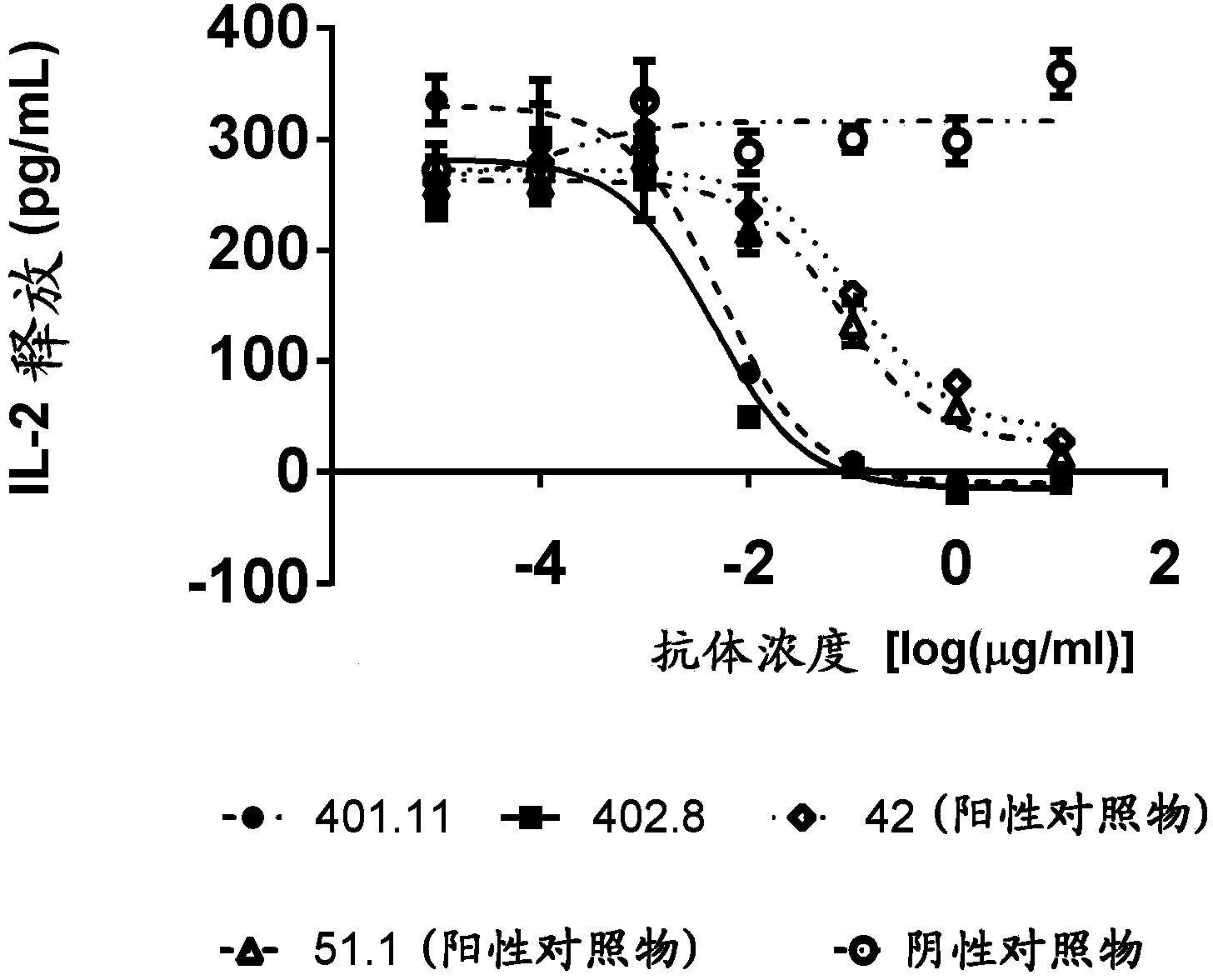
[0242] Example 3-Cell-based CD1d tetramer inhibition titer determination
[0243] Generate stable NKTCR expressing cell line
[0244] In order to develop a cell-based assay that characterizes the biological potency of anti-CD1d antibodies, a stable cell line expressing the NKT cell receptor (NKTCR) is required. The cell line J.RT3-T3.5 (ATCC:TIB-153) was selected to generate a stable NKT cell receptor expressing cell line. J. RT3-T3.5 is derived from the E6-1 clone of Jurkat lacking the β chain of the T cell antigen receptor (ATCC: TIB152). The cell does not express CD3 or the T cell receptor αβ heterodimer on the surface. J.RT3-T3.5 cells were electroporated with two vectors, one containing the α chain of J3N.5NKTCR (SEQ ID NO: 21), and the other containing the β chain of J3N.5NKTCR (SEQ ID NO: 22) (Brigl, M. et al., 2006 JImmunol 176:3625-34.). This NKT cell receptor is reactive to the glycolipid antigen α-GalCer. These vectors encoding the α and β chains of the NKTCR also e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com