Polyphenylene oxide anion exchange membrane for alkaline membrane fuel cells
An anion exchange membrane and fuel cell technology, which is applied in the field of polyphenylene ether anion exchange membrane and its preparation, can solve the problem of insufficient mechanical properties to meet the normal operation of the fuel cell, and achieves a simple and easy membrane preparation process, low cost and high performance. The effect of ion exchange capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 1.00g of BPPO in N-methylpyrrolidone, stir magnetically, and form a homogeneous solution, then add 0.1-50% (percentage of substance) of N,N of BPPO structural units (preferably 5-20%) ,N',N'-Tetramethyl-1,6-hexanediamine (TMHDA), heated at 50°C for 12h, and the N-methylpyrrolidone solution of partially cross-linked PPO obtained by TMHDA is denoted as T-PPO-I .
[0034] In the prepared T-PPO-I solution, add N-methylimidazole in a ratio of 1:1 to the amount of BPPO structural unit substance, react, the preferred reaction temperature is from room temperature to 100°C, and the reaction time is preferably 10min-2h, to obtain The solution is denoted as T-PPO-Im-I.
[0035]The prepared T-PPO-Im-I solution was placed in a flat-bottomed glass dish, dried to obtain a film, and soaked in 1molL at 20°C -1 NaOH solution, the immersion time is preferably 5-60h, after washing with deionized water, an alkaline T-PPO-Im-I membrane is obtained.
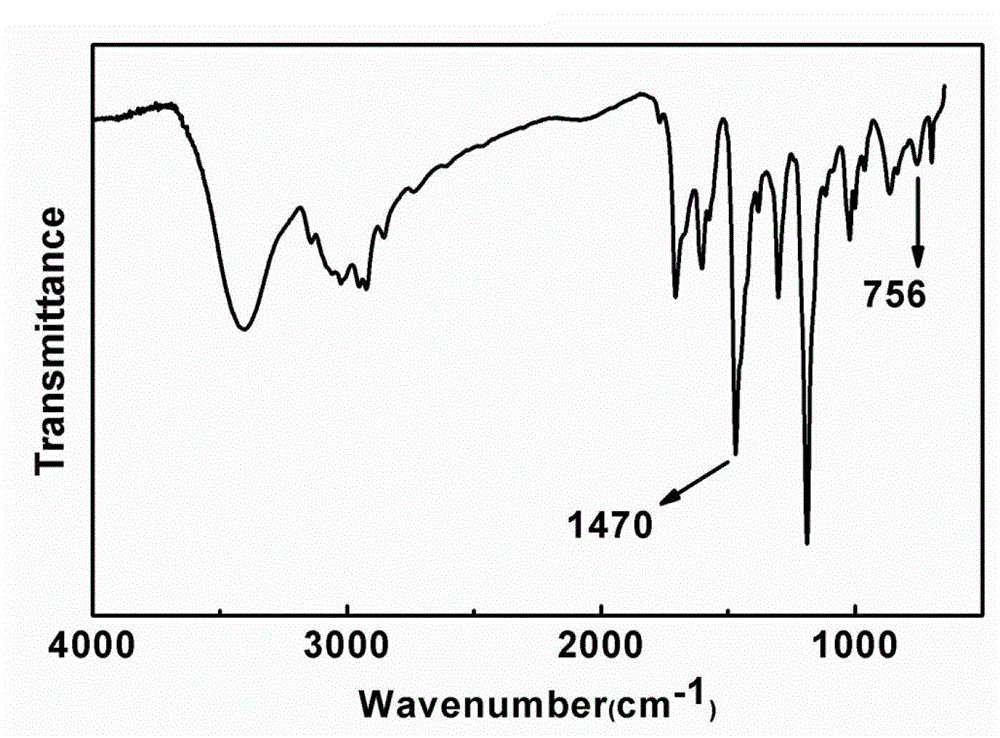
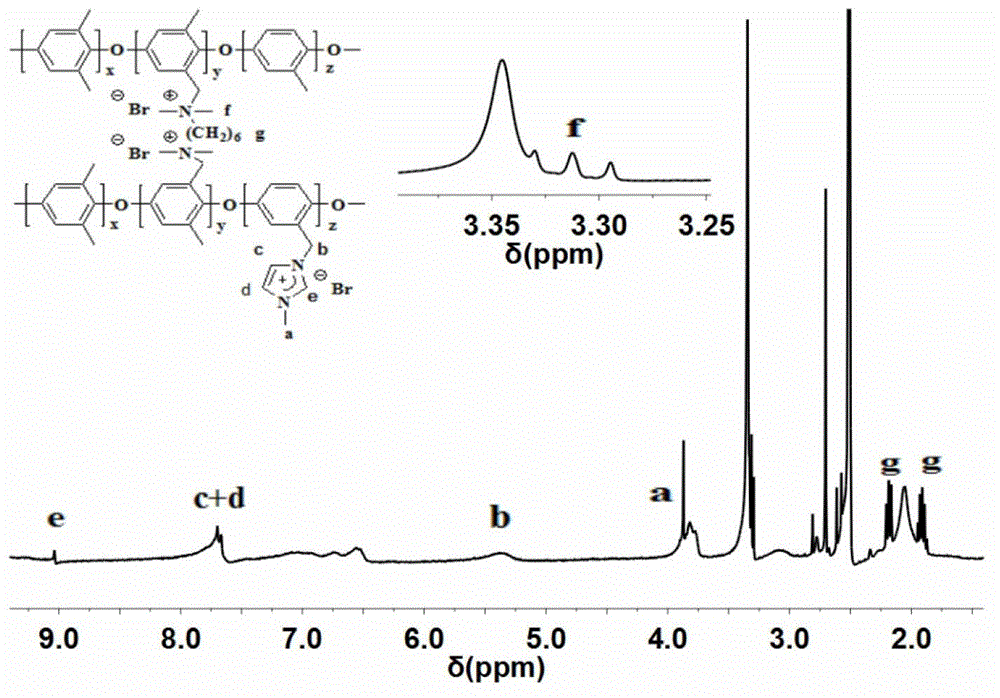
[0036] figure 1 It is the FT...
Embodiment 2
[0039] Dissolve 1.00g of BPPO in N-methylpyrrolidone, stir magnetically, and form a homogeneous solution, then add 0.1-50% (percentage of substance) of N,N of BPPO structural units (preferably 5-20%) ,N',N'-Tetramethyl-1,6-hexanediamine (TMHDA), heating reaction at 50°C, the reaction time is preferably 10-15h, and the obtained N-methylpyrrolidone solution of TMHDA partially cross-linked PPO, Recorded as T-PPO-II.
[0040] In the prepared T-PPO-II solution, add N-methylimidazole in a ratio of 1:1 to the amount of BPPO structural unit substance, react, preferably the reaction temperature is from room temperature to 100°C, and the reaction time is preferably 10min-2h, to obtain The solution is denoted as T-PPO-Im-II.
[0041] The prepared T-PPO-Im-II solution was placed in a flat-bottomed glass dish, dried to obtain a film, and soaked in 1mol L -1 NaOH solution, the immersion time is preferably 5-60h, after washing with deionized water, an alkaline T-PPO-Im-II membrane is obtai...
Embodiment 3
[0044] Dissolve 1.00g of BPPO in N-methylpyrrolidone, stir magnetically, and form a homogeneous solution, then add 0.1-50% (percentage of substance) of N,N of BPPO structural units (preferably 5-20%) , N',N'-tetramethyl-1,6-hexanediamine (TMHDA), heating reaction, the reaction temperature is preferably 40-60 ° C, the time is preferably 10-15h, the obtained TMHDA partially cross-linked PPO N -Methylpyrrolidone solution, denoted as T-PPO-III.
[0045] In the prepared T-PPO-III solution, add N-methylimidazole in a ratio of 1:1 to the amount of BPPO structural unit substance, and react, preferably the reaction temperature is from room temperature to 100°C, and the reaction time is preferably 10min-2h, to obtain The solution is denoted as T-PPO-Im-III.
[0046] The prepared T-PPO-Im-III solution was placed in a flat-bottomed glass dish, dried to obtain a film, and soaked in 1mol L -1 NaOH solution, the immersion time is preferably 5-60h, after washing with deionized water, an alk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com