Kit for detecting VKH (Vogt Koyanagi Harada) syndrome
A kit and syndrome technology, which is applied in the field of kits for detecting VKH syndrome, can solve the problems of rare non-HLA antigen-related reports, and achieve the effect of preventing the development of lesions and correct diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Sample collection and extraction of genomic DNA
[0030] The samples came from the First Affiliated Hospital of Chongqing Medical University. A total of 1,559 VKH patients were collected, including 55.0% males and 45.0% females; 5,640 normal controls, 50.8% males and 49.2% females. All subjects were Han nationality, and voluntarily signed the informed consent, and this study was also approved by the ethics committee.
[0031] Genomic DNA from human peripheral blood was prepared according to the operating steps provided in the instruction manual of the QIAGEN QIAamp DNA Mini Blood Kit kit (purchased from QIAGEN, Germany).
Embodiment 2
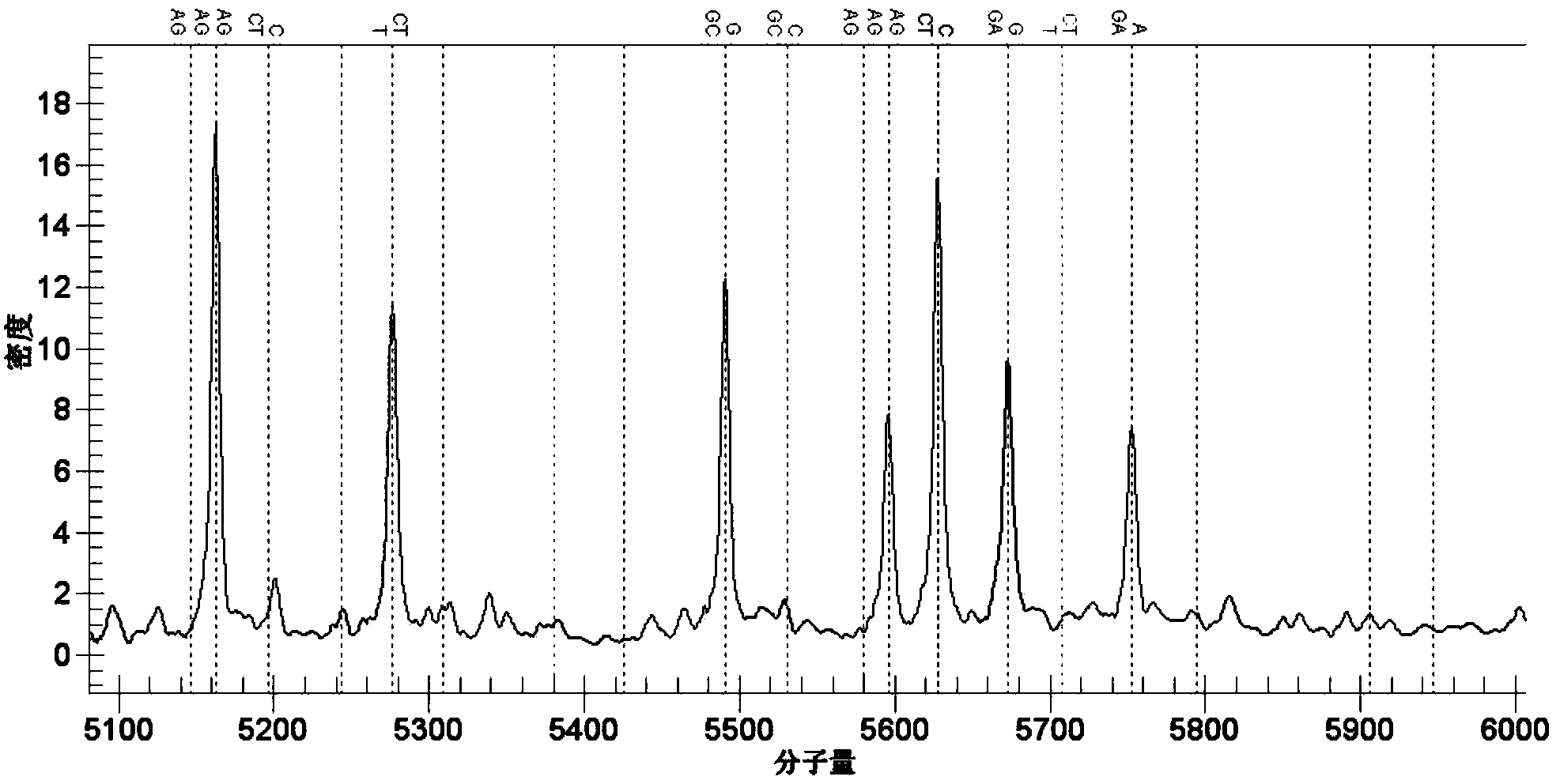
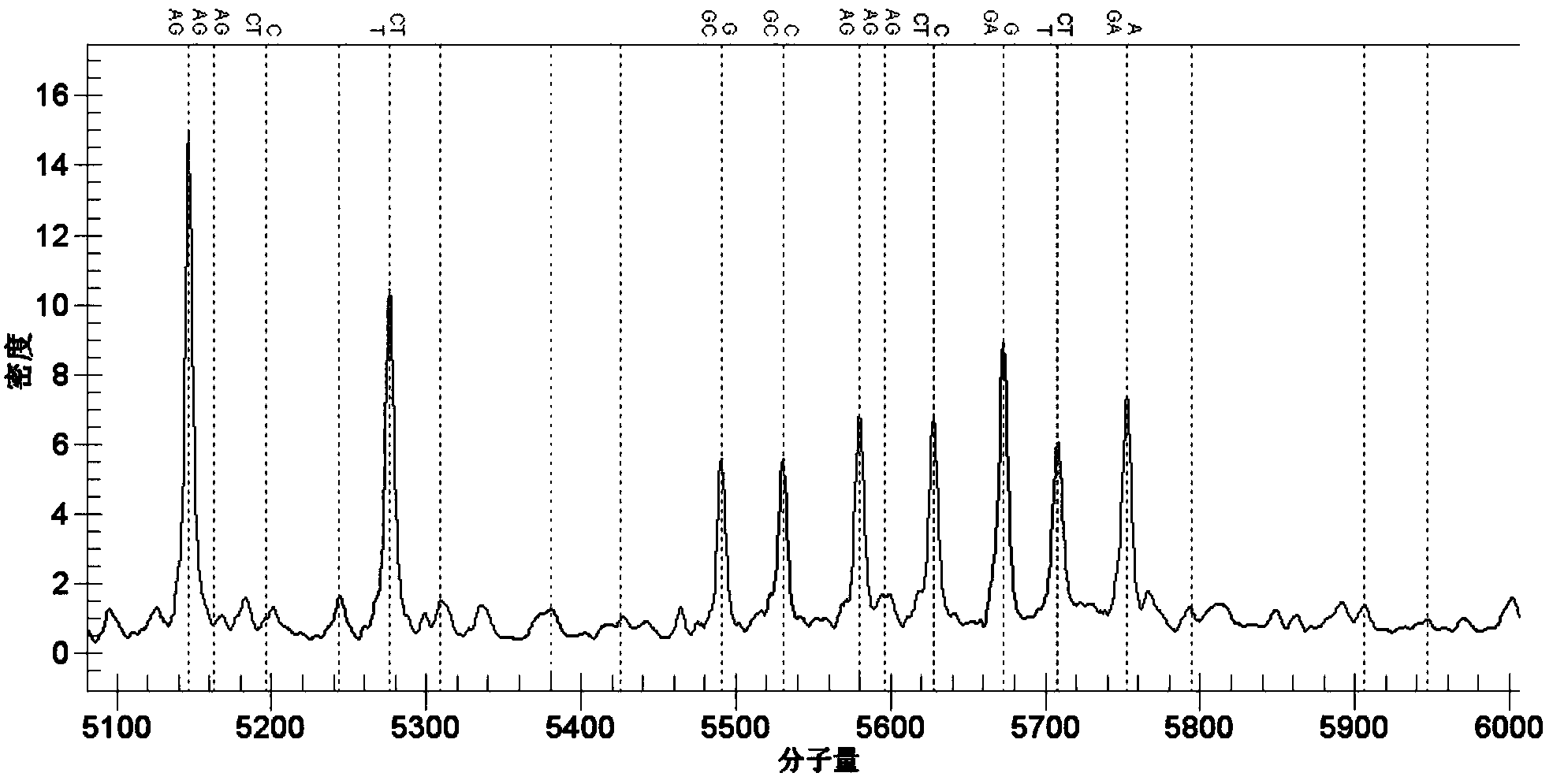
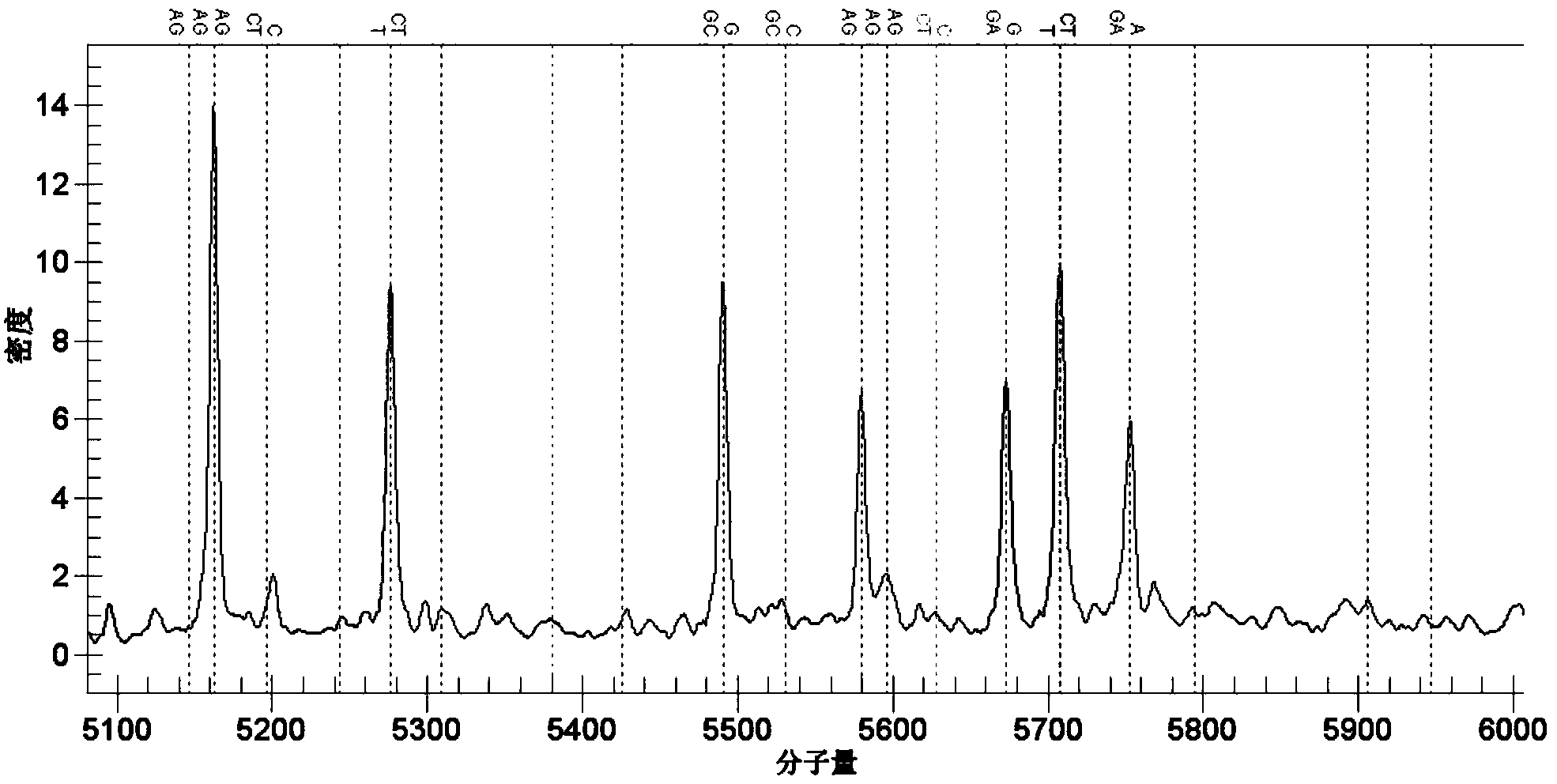
[0032] 实施例2包括rs78377598、rs77258390、rs78597810、rs12568393、rs12561798、rs76436269,[rs117633859、rs12564219、rs12563505、rs34017352、rs114661385、rs117301158、rs115009271、rs76091599、rs78865162、rs78917656、rs6693659、rs12566159、rs117282985];rs3021304[rs114800139];rs442309 PCR Amplification and Genotype Analysis of Single Nucleotide Polymorphic DNA Fragments of and rs224058 [rs224048, rs224052, rs224057, rs10995307, rs10995281, rs7088592, rs224071, rs224031, rs224032, rs224033]
[0033] The present invention uses the Sequenom MassARRAY SNP detection technology to detect the mutations of rs78377598, rs77258390, rs78597810, rs12568393, rs12561798, rs76436269, rs3021304, rs442309 and rs224058 (as shown below).
[0034] 1) Use SEQ ID NO.1~2, SEQ ID NO.3~4, SEQ ID NO.5~6, SEQ ID NO.7~8, SEQ ID NO.9~10, SEQ ID NO.11~12 , the primers shown in SEQ ID NO.13-14, SEQ ID NO.15-16, and SEQ ID NO.17-18 carry out target gene fragments containing specific sites;
[0035] PCR amplification: 94°C for 4min; ...
Embodiment 4
[0125] Embodiment 4 detection kit
[0126] All components, content and method of use in the kit of the present invention are as follows:
[0127] PCR amplification reagent (50 servings)
[0128] Wherein, the primer pair A-I are the primers shown in SEQ ID NO.1-2-SEQ ID NO.19-20 respectively. Primers A-I are PCR amplification primers, and a-i are specific extension primers.
[0129]
components
concentration
volume
Taq enzyme
4ml
SAP (Shrimp Alkaline Phosphatase)
80ul
SAP Buffer
10×
60ul
Single base extension reaction solution
500ul
PCR primer pair A (rs78377598)
10uM
400ul
PCR primer pair B (rs77258390)
10uM
400ul
PCR primer pair C (rs78597810)
10uM
400ul
PCR primer pair D (rs12568393)
10uM
400ul
PCR primer pair E (rs12561798)
10uM
400ul
PCR primer p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com