Polymorphous Forms III And IV Of N-Benzoyl - Staurosporine
A technology of benzoyl star and crystal form, which is applied in the direction of medical preparations containing active ingredients, antibacterial drugs, drug combinations, etc., and can solve problems such as difficult application of melt extrusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
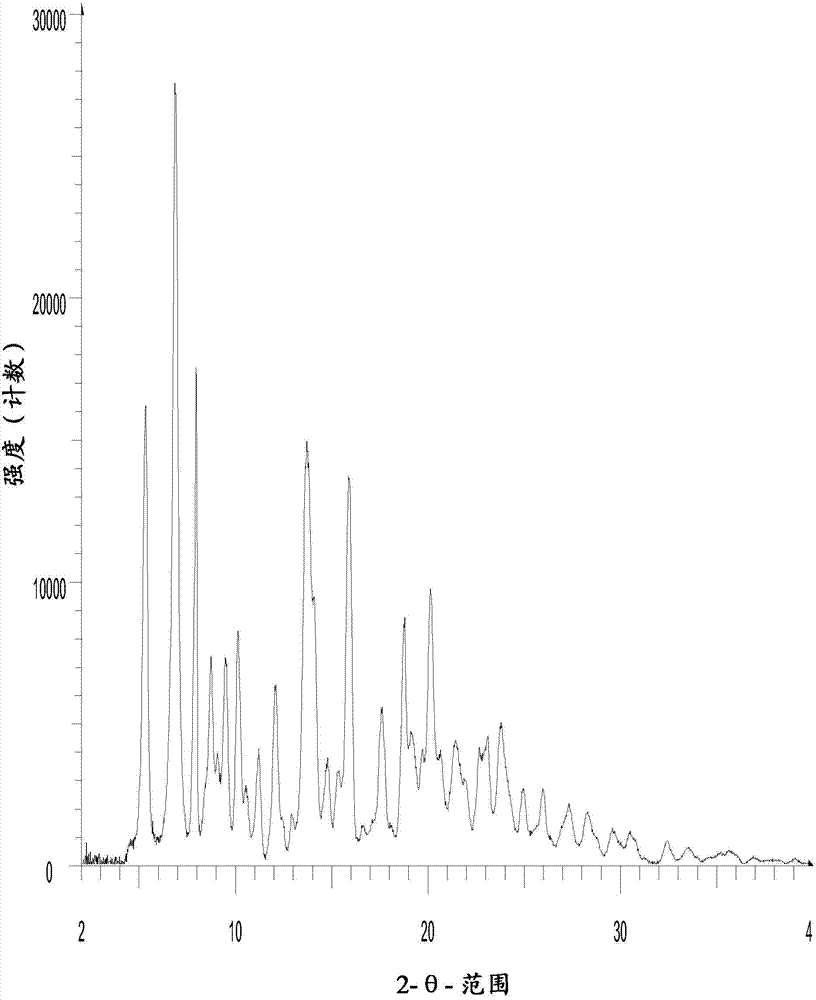
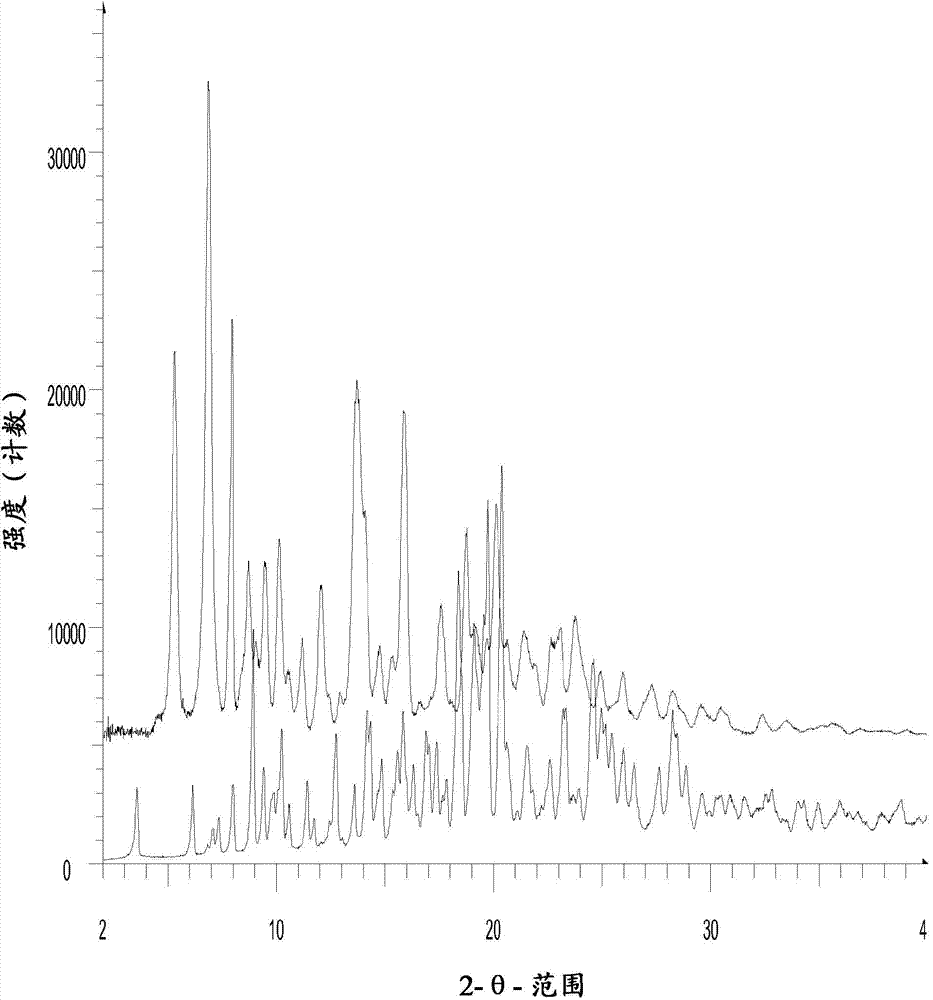
[0087] In Example 1, Form III of N-benzoylstaurosporine was prepared by GAS recrystallization. The X-ray diffraction pattern of the crystal form III is in figure 1 , and the X-ray diffraction patterns of Form III and Form II disclosed in WO 2006 / 048296 are in figure 2 displayed in .
Embodiment 2
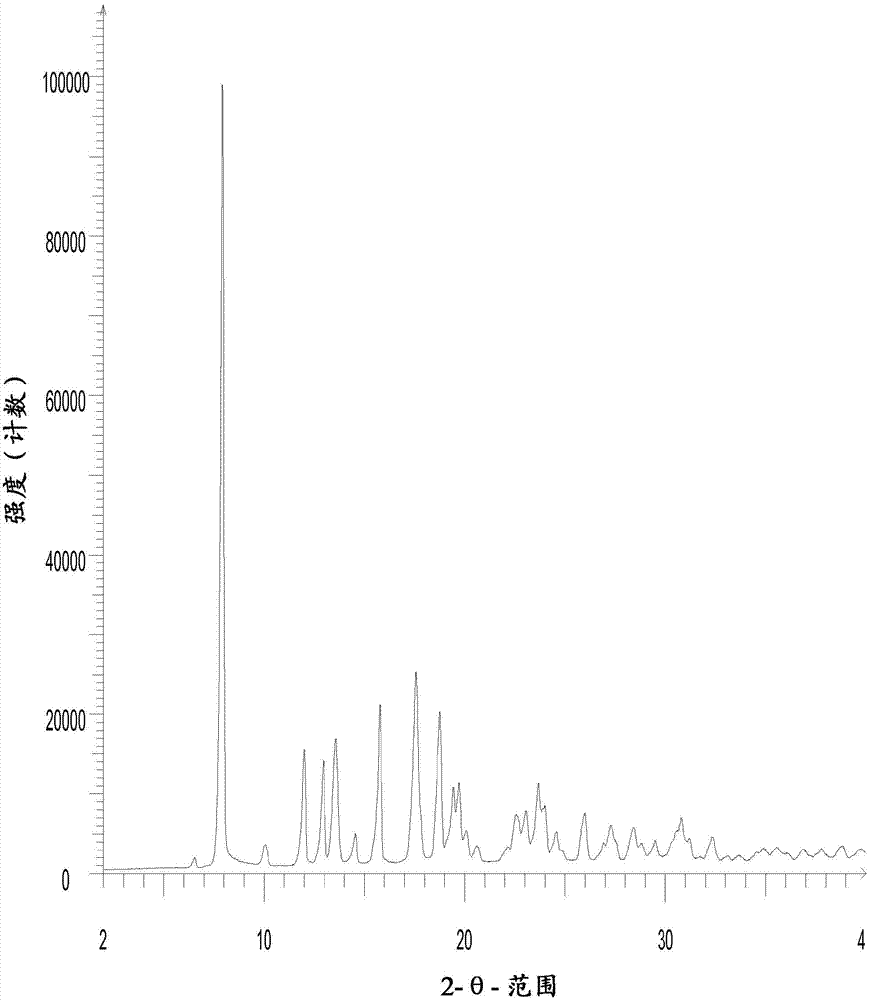
[0089] In Example 2, crystalline form IV of N-benzoylstaurosporine was prepared by GAS recrystallization. The X-ray diffraction pattern of crystal form IV is in image 3 , and the X-ray diffraction patterns of Form IV and Form II disclosed in WO 2006 / 048296 are in Figure 4 displayed in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com