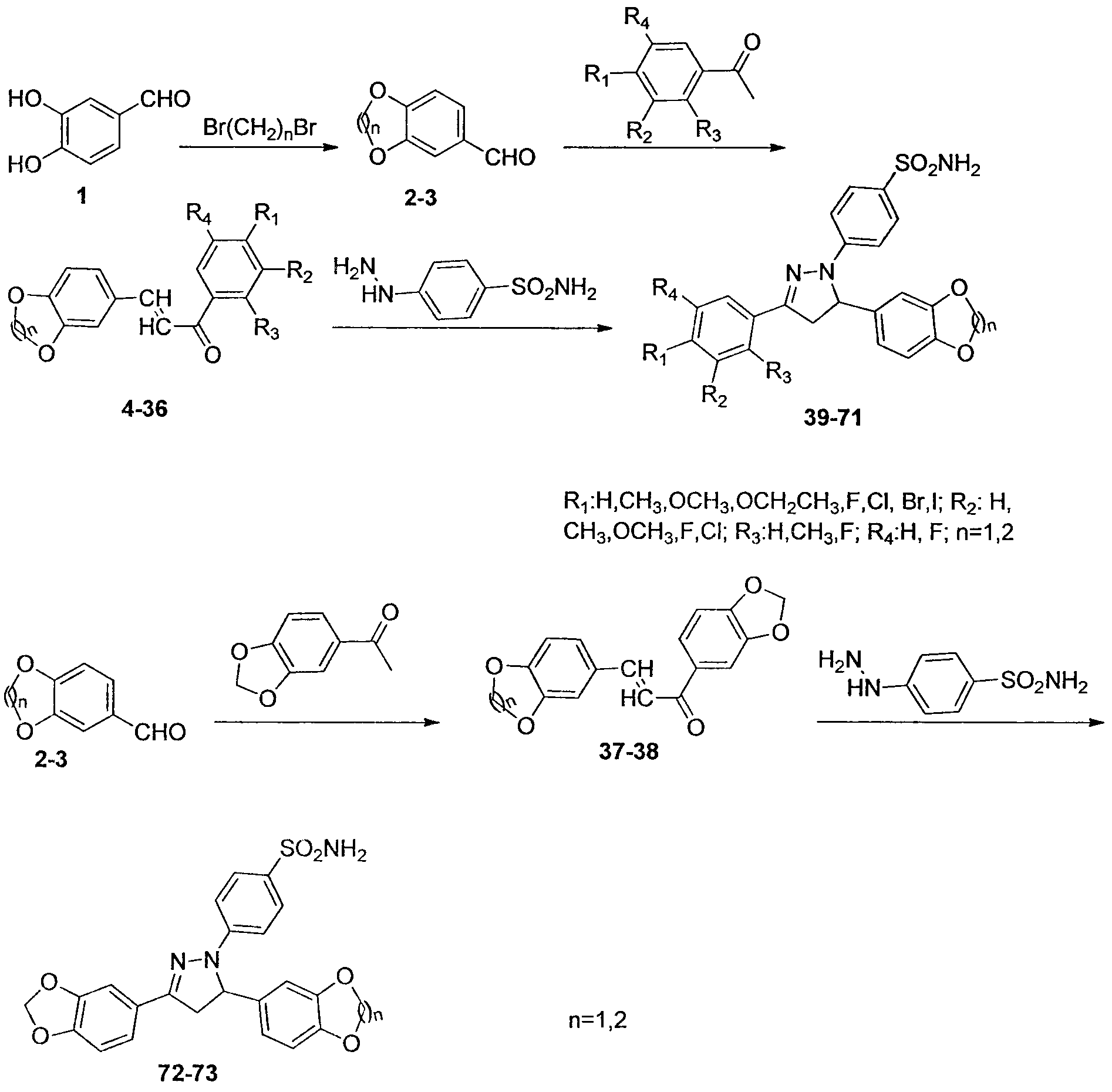
Synthesis of dihydropyrazol sulfonamide derivatives containing benzodioxane skeletons and application of dihydropyrazol sulfonamide derivatives in anti-cancer drugs
A technology of dihydropyrazolesulfonamide and benzodioxane, which is applied in the synthesis of a class of dihydropyrazolesulfonamide derivatives containing benzodioxane skeleton and its application in anticancer drugs, can solve the problem of easy Problems such as drug resistance, application range limitation, and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
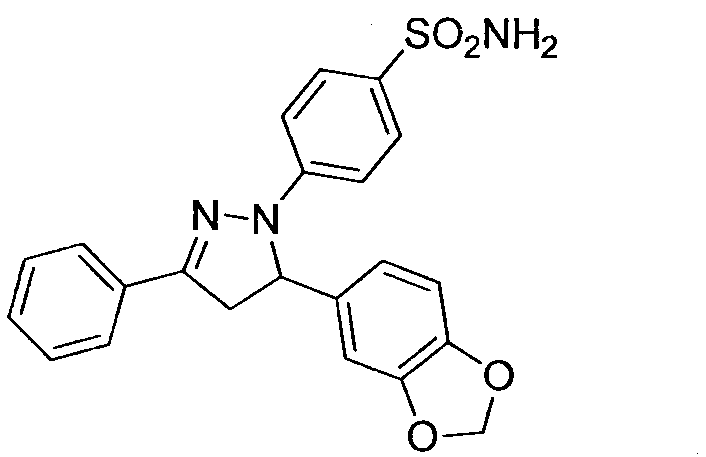
[0014] Example 1: Preparation of 4-(5-(1.3-benzodioxane)-3-phenyl-4,5-dihydropyrazole)benzenesulfonamide (compound 39)
[0015]
[0016] Under stirring, 1,3-epoxybenzodioxanechalcone (1.0g, 3.87mmol), ethanol (25mL), p-hydrazinobenzenesulfonamide (0.97g, 5.03mmol), acetic acid (1.0mL ) into a 50mL round-bottomed flask, some solids were still insoluble; the flask was transferred to an oil bath, refluxed for 6h, and TLC tracked the reaction (developing agent V AcOEt :V 正己烷 =1:2), after the reaction was completed, filtered, the solid was washed with distilled water, and finally dried in vacuum, the obtained solid was dissolved in absolute ethanol for recrystallization and purification to obtain the crystalline target compound.
[0017] White crystals, yield 66.4%. m.p.212~214℃; 1 H NMR (DMSO-d6, 400MHz) δ: 7.80 (d, J=7.4Hz, 2H, ArH), 7.62 (d, J=8.6Hz, 2H, ArH), 7.48~7.40 (m, 3H, ArH), 7.11 (d, J=8.6Hz, 2H, ArH), 7.04 (s, 2H, NH 2 ), 6.87(d, J=7.9Hz, 1H, ArH), 6.78~6.75(m,...
Embodiment 2
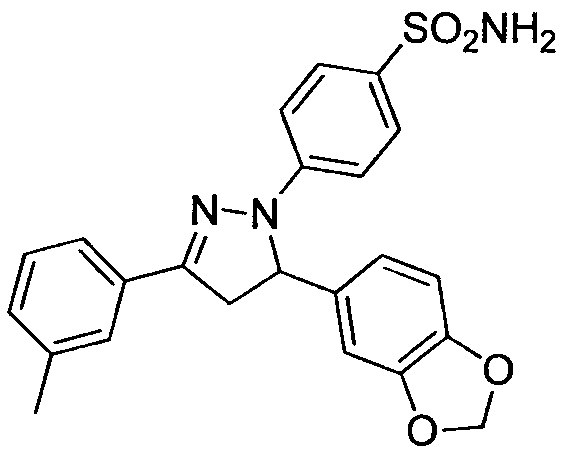
[0018] Example 2: Preparation of 4-(5-(1.3-benzodioxane)-3-(m-methylphenyl-4,5-dihydropyrazole)benzenesulfonamide (compound 40)
[0019]
[0020] The preparation method is the same as in Example 1. White crystals were obtained with a yield of 75.8%. m.p.187~188℃; 1 H NMR (DMSO-d6, 400MHz) δ: 7.64~7.56 (m, 4H, ArH), 7.34 (t, J=7.6Hz, 1H, ArH), 7.23 (d, J=7.4Hz, 1H, ArH), 6.10 (d, J=8.6Hz, 2H, ArH), 7.04 (s, 2H, NH 2 ), 6.87(d, J=7.8Hz, 1H, ArH), 6.75(d, J=9.9Hz, 2H, ArH), 5.98(s, 2H, CH 2 ), 5.55 (dd, J 1 =4.7,J 2 = 4.8Hz, 1H, 5-H), 3.91(dd, J 1 =12.1,J 2 = 12.1Hz, 1H, 4-H b ), 3.17 (dd, J 1 =4.8,J 2 =4.7Hz, 1H, 4-Ha), 2.37(s, 3H, CH 3 ), .ESI-MS: 436.1 [M+H] + .Anal.Calcd for C 21 h 23 N 3 o 4 S: C, H, N.
Embodiment 3
[0021] Example 3: Preparation of 4-(5-(1.3-benzodioxane)-3-(m-methoxyphenyl-4,5-dihydropyrazole)benzenesulfonamide (compound 41)
[0022]
[0023] The preparation method is the same as in Example 1. White crystals were obtained with a yield of 76.6%. m.p.219~221℃; 1 H NMR (DMSO-d6, 400MHz) δ: 7.61 (d, J=8.7Hz, 2H, ArH), 7.37~6.99 (m, 8H, ArH), 6.87 (d, J=7.9Hz, 1H, ArH), 6.76 (s, 2H, NH 2 ), 6.00 (s, 2H, CH 2 ), 5.56 (dd, J 1 =4.9,J 2 =5.0Hz, 1H, 5-H), 3.91(dd, J 1 =12.2,J 2 =12.0Hz, 1H, 4-H b ), 3.82 (s, 3H, CH 3 ), 3.18 (dd, J 1 =5.0,J 2 = 4.8Hz, 1H, 4-Ha).ESI-MS: 452.1 [M+H] + .Anal.Calcd for C 21 h 23 N 3 o 5 S: C, H, N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com