Bi-zinc-phthalocyanine coordination compound and preparation method and application thereof
A technology of diphthalocyanine zinc and complexes, which is applied in the field of diphthalocyanine zinc complexes and their preparation, and can solve the problem of low tumor targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Correspondingly, the embodiment of the present invention also provides a preparation method of the above-mentioned ZnPc-S-S-ZnPc. The method comprises the steps of:
[0030] S01: Preparation of reaction solution: dissolve β-monocarboxyl substituted zinc phthalocyanine and 2,2'-dithiodiethanol in an organic reaction solvent, cool down to below 5°C, and then add condensing agent, condensation activator and catalyst to obtain Reactant mixed solution;
[0031] S02: make the reaction solution react: the step reactant mixed solution is warmed up to 30~60 ℃ to carry out condensation reaction until the end of the reaction; ) shown in the zinc diphthalocyanine complex,
[0032]
[0033]Specifically, in the above step S01, the molecular structural formula of β-monocarboxyl substituted zinc phthalocyanine is as compound a in the following reaction formula (2), which can be combined with 2,2'-disulfide in the reaction system of the above step S02 Ethanol undergoes a condensat...
Embodiment 1
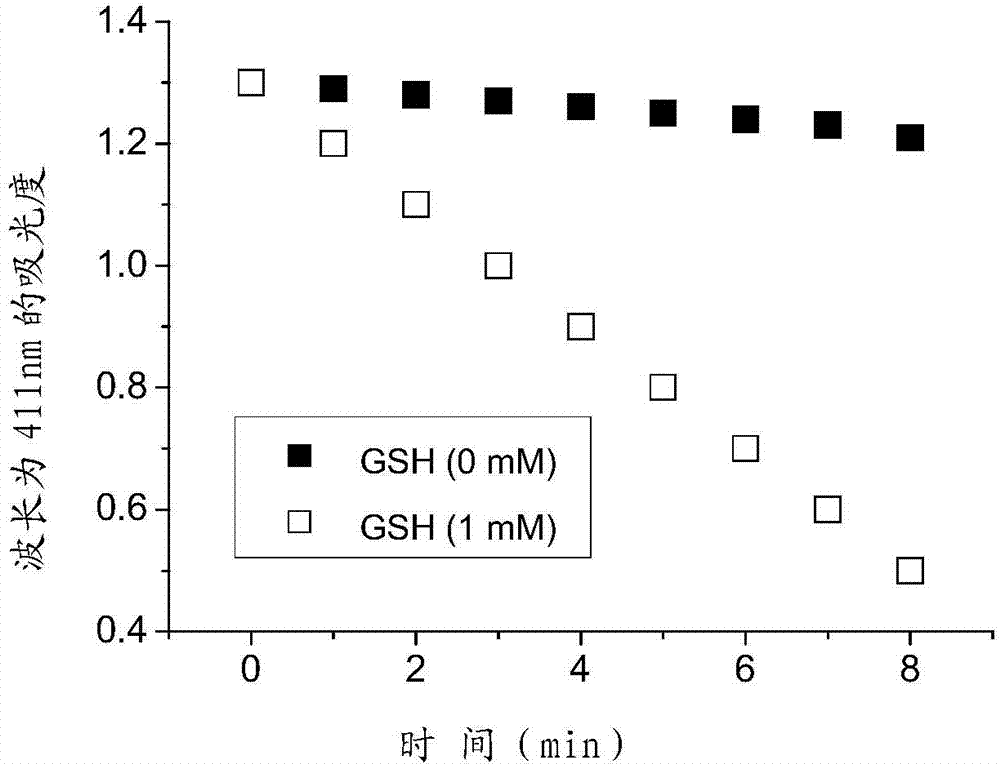
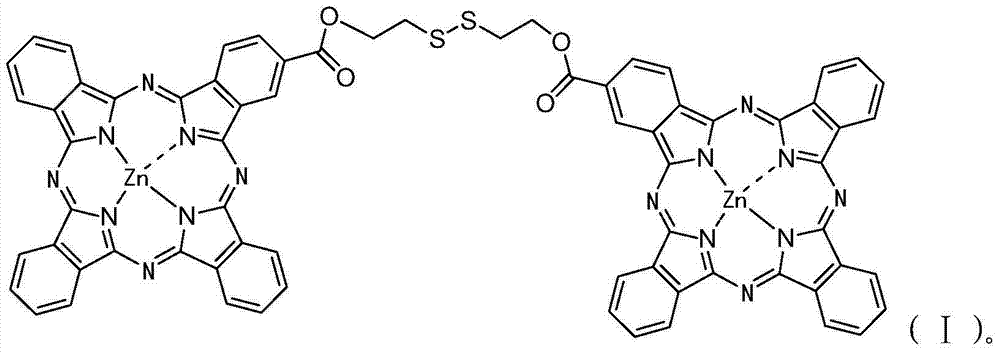
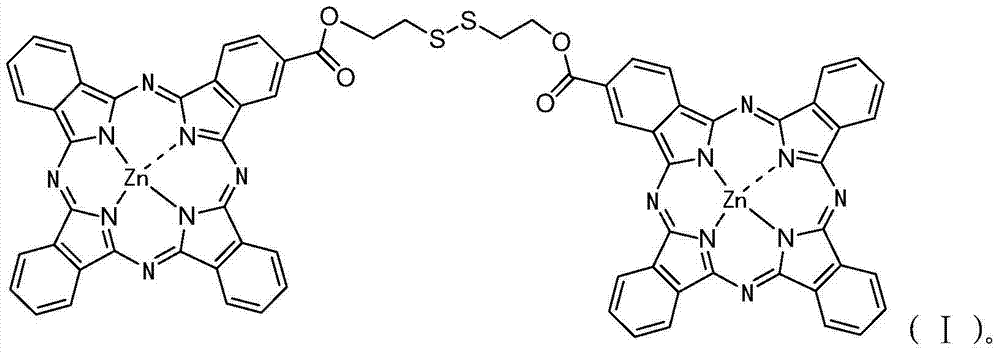
[0049] Diphthalocyanine zinc complex (ZnPc-S-S-ZnPc) and its synthesis method. The molecular structural formula of the zinc diphthalocyanine complex (ZnPc-S-S-ZnPc) is shown in formula (I) above.
[0050] Its synthesis method is as follows:
[0051] Dissolve β-monosubstituted phthalocyanine zinc complex (620mg, 1mmol) and 2,2'-dithiodiethanol (154mg, 1mmol) in N,N'-dimethylformamide (50mL), and the temperature of the solution was reduced to Stir at 0°C for 0.5 hours, slowly add dicyclohexylcarbodiimide (824mg, 4mmol), N-hydroxysuccinimide (460mg, 4mmol), 4-N,N-lutidine (244mg, 2mmol) , the temperature of the reaction solution was raised to 60° C. for 12 hours. The reaction solution was cooled to room temperature, filtered, the yield filtrate was dried under reduced pressure to remove the solvent, and the remaining solid was separated and purified by silica gel chromatography, and the analytical agent was chloroform / methanol (10:1v / v) to collect the main components and dried ...
Embodiment 2
[0054] Diphthalocyanine zinc complex (ZnPc-S-S-ZnPc) and its synthesis method. The molecular structural formula of the zinc diphthalocyanine complex (ZnPc-S-S-ZnPc) is shown in formula (I) above.
[0055] Its synthesis method is as follows:
[0056] Dissolve β-monosubstituted zinc phthalocyanine complex (620mg, 1mmol) and 2,2'-dithiodiethanol (77mg, 0.5mmol) in acetonitrile (50mL), lower the temperature of the solution to 5°C and stir for 0.5 hours, slowly Add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (382mg, 2mmol), 1-hydroxybenzotriazole (270mg, 2mmol), triethylamine (404mg, 4mmol ), the temperature of the reaction solution was raised to 50°C for 20 hours. The reaction solution was cooled to room temperature, filtered, the yield filtrate was dried under reduced pressure to remove the solvent, and the remaining solid was separated and purified by silica gel chromatography. The analytical agent was dichloromethane / ethanol (10:1), and the main components were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com