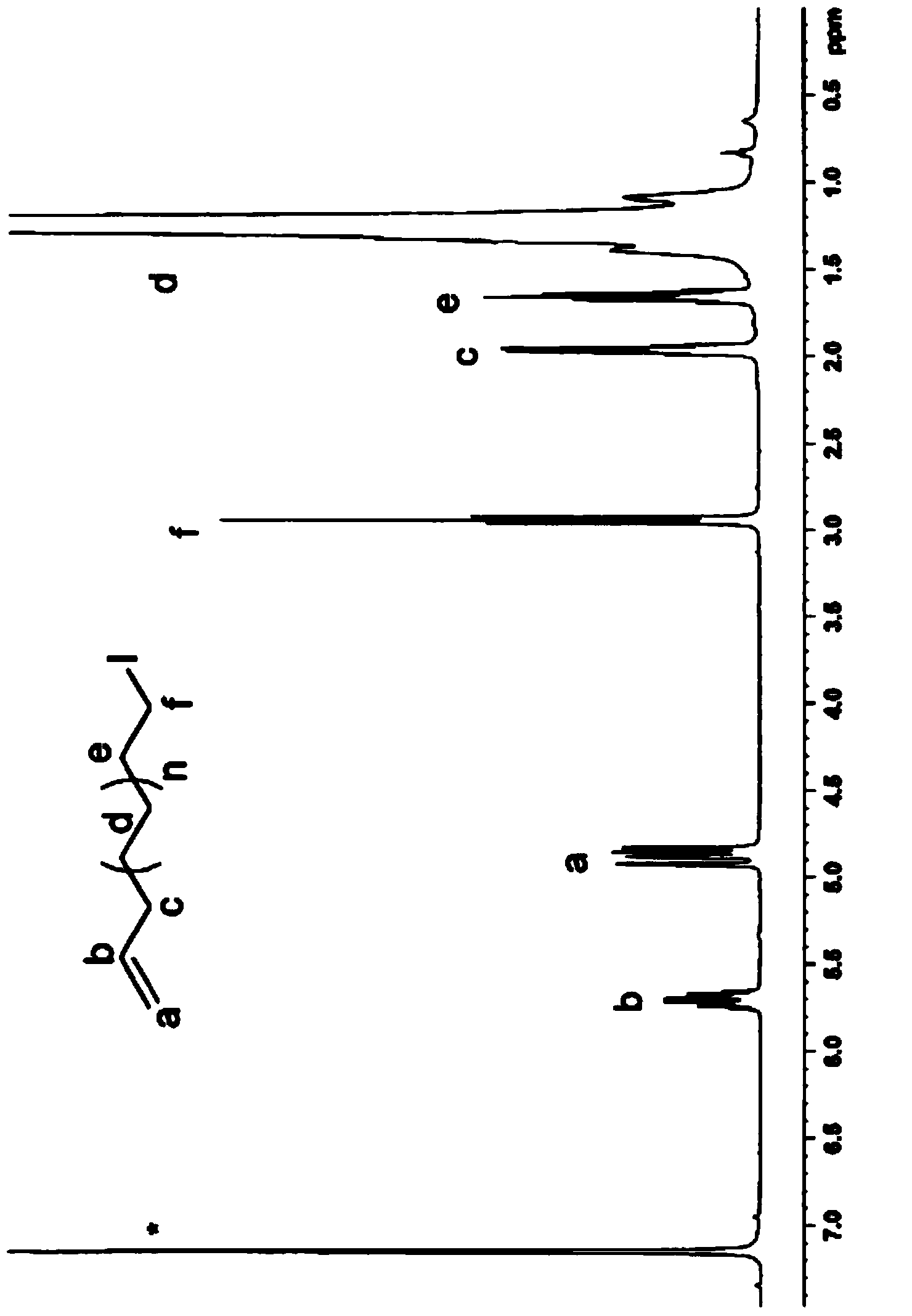
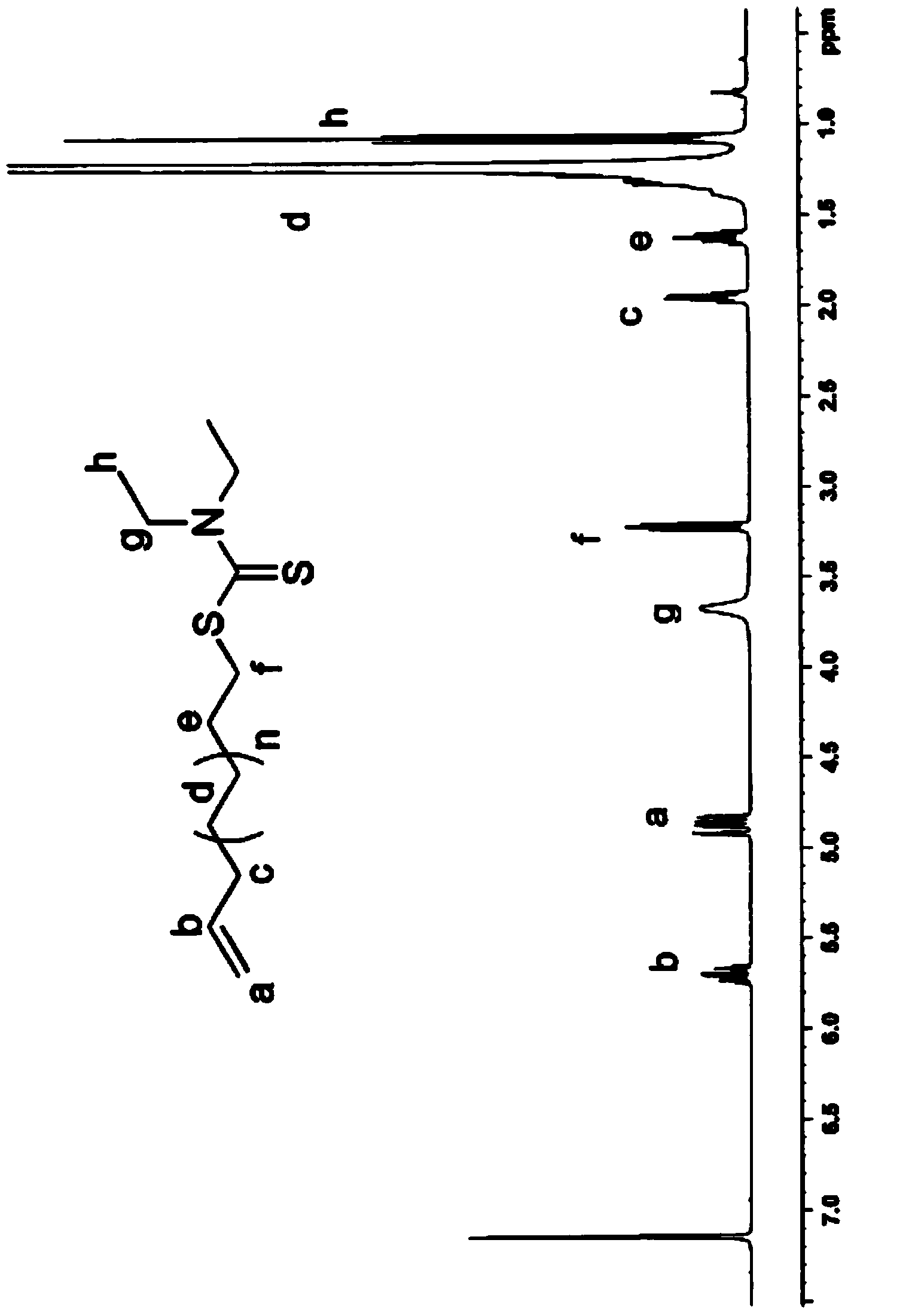
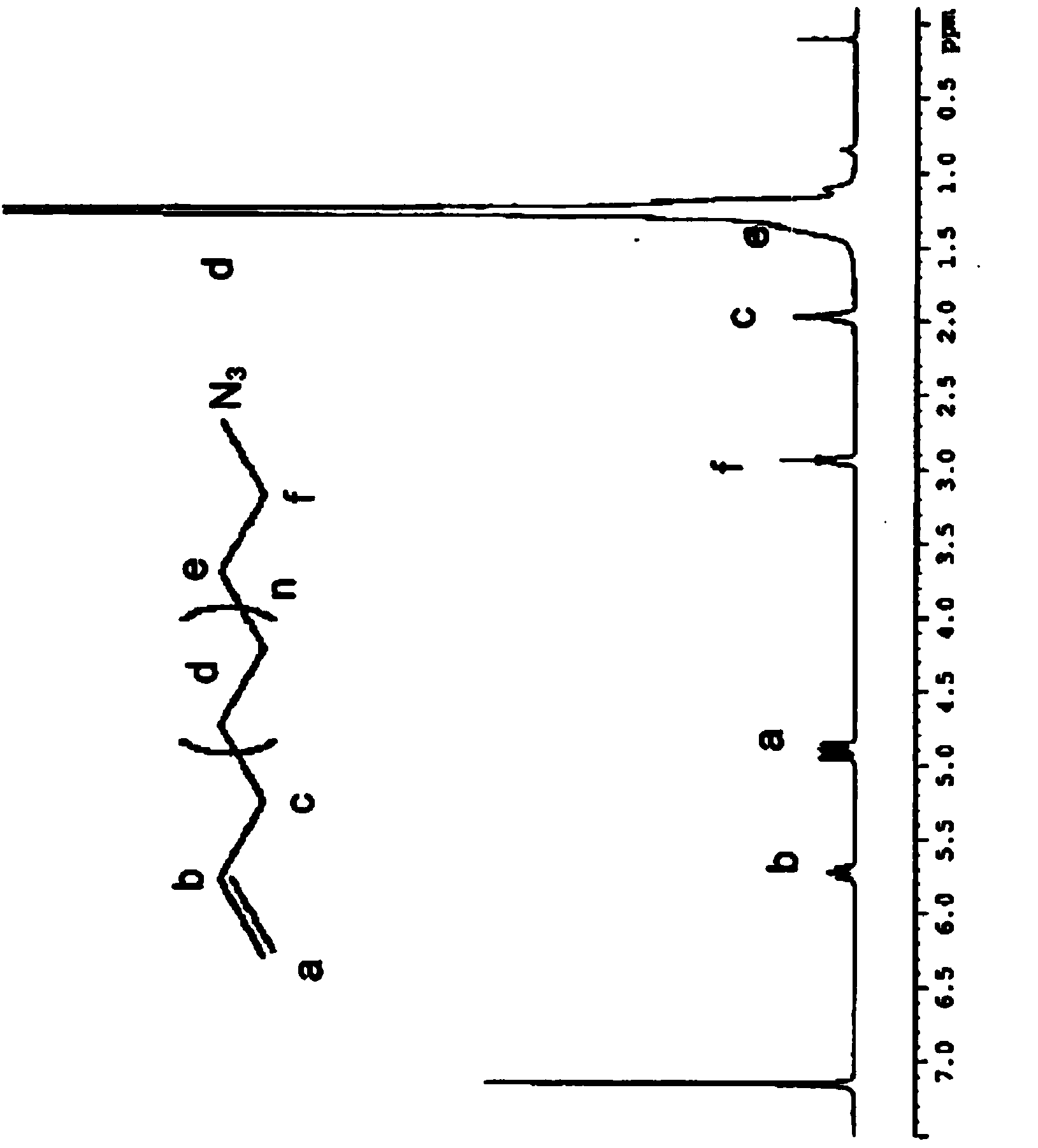
Telechelic polyolefin and preparation thereof
A polyolefin, telechelic technology, applied in the fields of compounds containing elements of Group 3/13 of the periodic table, organic chemistry, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0078] The following examples relate to the preparation:
[0079] - a transfer agent of formula (III);
[0080] - an intermediate compound of formula (II);
[0081] - A telechelic polyolefin of formula (I).
[0082] Transfer agent bis(10-undecenyl)magnesium (Mg((CH 2 ) 9 CH=CH 2 ) 2 )Synthesis
[0083] To a suspension of magnesium (2.38 g, 98 mmol) in di-n-butyl ether (100 ml) was added 11-bromo-1-undecene (11.3 ml, 49 mmol) dropwise at 0°C. The reaction mixture was stirred at 0°C for 1 hour and then allowed to warm to room temperature. The resulting suspension was filtered to remove excess magnesium. Dioxane (5.0ml, 59mmol) was added to the filtrate, immediately after which a white precipitate formed. The suspension was stirred for 2 hours, then filtered to obtain Mg((CH 2 ) 9 CH=CH 2 ) 2 Solution in di-n-butyl ether. An aliquot was taken from the solution for concentration determination by titration using i) pyrene-1-acetic acid and ii) HCl(aq) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com