Favipiravir pharmaceutical composition containing different particle size ranges
A favipiravir and composition technology, which is applied in the field of favipiravir pharmaceutical compositions, can solve problems such as inability to measure dissolution rate, impact, and poor solubility of favipiravir, so as to improve the bioavailability of human body and quickly release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
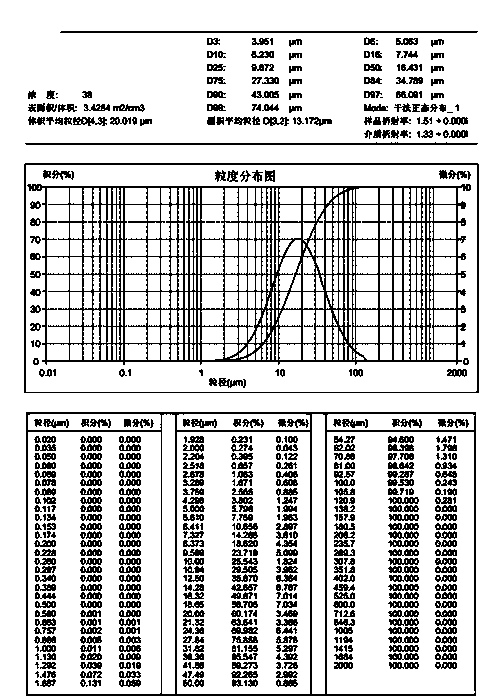
[0040] Sampling according to prescription 1, jet milling Favipiravir, collecting materials, and measuring particle size distribution (dry method measurement), requiring D10=0.1~8 microns, D50=10~30 microns, D90<50 microns. The lactose monohydrate, microcrystalline cellulose, and corn starch are pulverized to control the particle size distribution D90<75 microns respectively. Mix lactose monohydrate, microcrystalline cellulose, and cornstarch in multiple mobile mixers for 20 minutes, take 10 samples from different positions of the mixer, and measure the material uniformity. The RSD value is required to be less than 1.0%. Put the material that meets the requirements on the dry granulator, adjust the rotation speed of the extrusion wheel (3.6~5.3rpm), the rotation speed of the feeding screw (12~24rpm), the pressure of the oil cylinder (the pressure of the oil cylinder is 1.0 MPa~3.0 MPa), so that it can be three Those who cooperate effectively until the hardness of the pressed me...
Embodiment 2-4
[0042] Sampling according to prescription 2-4, method is the same as embodiment 1.
Embodiment 5
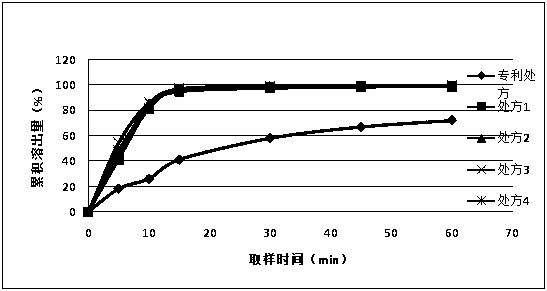
[0044] Dissolution test of Favipiravir in water
[0045] Dissolution test method: paddle method, 50 rpm, 900ml of water and acetate buffer (pH4.5). Measured by UV method, the wavelength is 323nm, and the concentration of the reference substance is about 8μg / ml.
[0046] From figure 1 It can be seen that at 15 minutes, the cumulative stripping is less than 50%, and only about 70% at 60 minutes, which is very different from the stripping of more than 95% in 15 minutes in the patent literature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com