Vaccine composition, preparation method and application thereof
A vaccine composition and antigen technology, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of not removing the immunosuppressive components of Mycoplasma hyopneumoniae, not using it, and cumbersome operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of Mycoplasma hyopneumoniae
[0067] 1.1. Preparation of whole bacterial antigen of Mycoplasma hyopneumoniae
[0068] After unsealing the freeze-dried strain Mycoplasma hyopneumoniae HN0613, inoculate the liquid culture medium with 10% of the inoculum amount, shake culture at 37°C for 3 to 7 days, and harvest when the pH value drops from 7.5 to 6.8. seed. Take the first-grade seeds and inoculate the liquid medium with 5% inoculum amount, shake and culture at 37°C for 3-7 days, harvest when the pH value drops from 7.5 to 6.8, and use them as secondary production seeds after pure inspection. Inoculate the secondary seeds of qualified Mycoplasma hyopneumoniae HN0603 strain in liquid culture medium at 5% (v / v) respectively, shake and culture at 37°C for 3-7 days, and harvest when the pH value drops from 7.5 to 6.8. Make the final concentration of formaldehyde solution 0.3% (V / V), inactivate at 37°C for 24 hours, stir once every 4 hours, 10min each ti...
Embodiment 2
[0078] The preparation of embodiment 2 swine mycoplasma pneumonia vaccine composition
[0079] The mycoplasma hyopneumoniae antigen prepared by embodiment 1 and Montanide TM Gel 01 adjuvant is mixed together by the components and ratio contained in the mycoplasma pneumoniae vaccine composition in Table 3, stirred at a speed of 500-800r / min for 10-15min, and added 1% (volume ratio) thimerosal solution before terminating the stirring , so that the final concentration does not exceed 1 / 10,000, fully shake and mix, and store at 2-8°C after aliquoting.
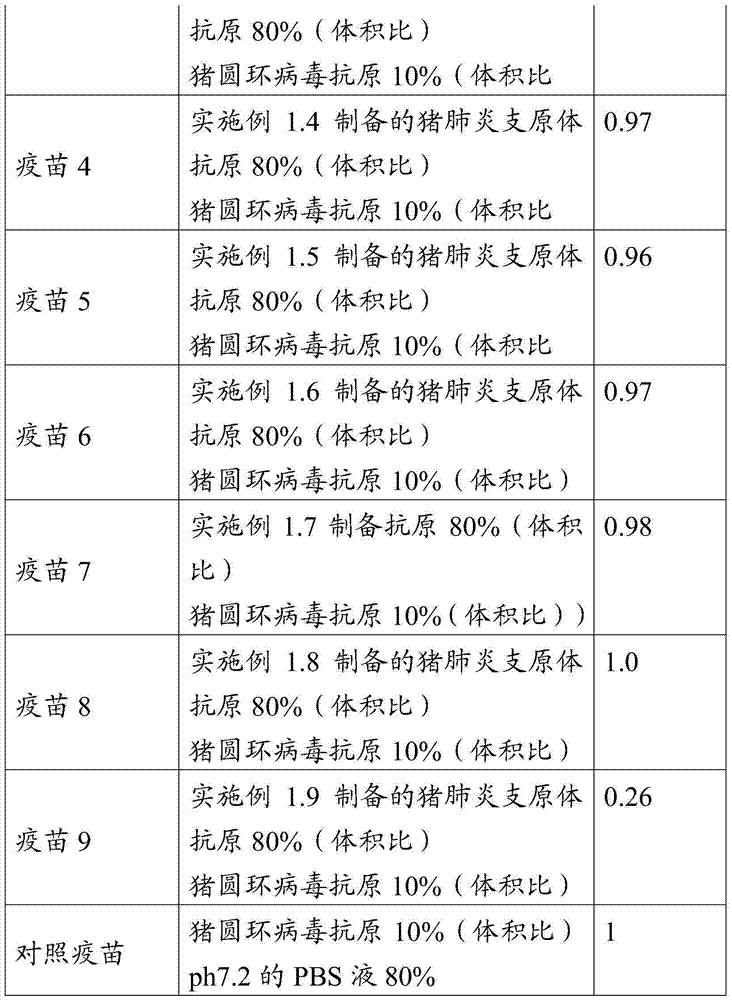
[0080] Table 1 Preparation of Mycoplasma hyopneumoniae vaccine composition
[0081]
Embodiment 3
[0082] Example 3 Efficacy Test of Vaccine Compositions of Mycoplasma Pneumoniae Porcine with Different Components
[0083] The vaccine composition prepared in Example 2 was used to immunize 50 piglets at the age of 14-21 days respectively (excluding porcine reproductive and respiratory syndrome, porcine circular type 2 and swine fever), totally 12 groups, 5 piglets / group, 1mL / head. 42 days after immunization, inject 100 MID of virulent CVCC354 strain of Mycoplasma hyopneumoniae Jinan strain into all pig trachea 50 / head, observed 28 days after dissection, and observed lung lesions, lung lesions were scored according to the 28-point method. About 10 days after the challenge, pigs in the blank control group (3 of them) successively developed symptoms such as coughing and wheezing, and their fur was not smooth;
[0084] Table 2 The scores of lung lesions and the reduction rate of lung lesions in each test group
[0085] Group No
[0086] The test results showed tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com