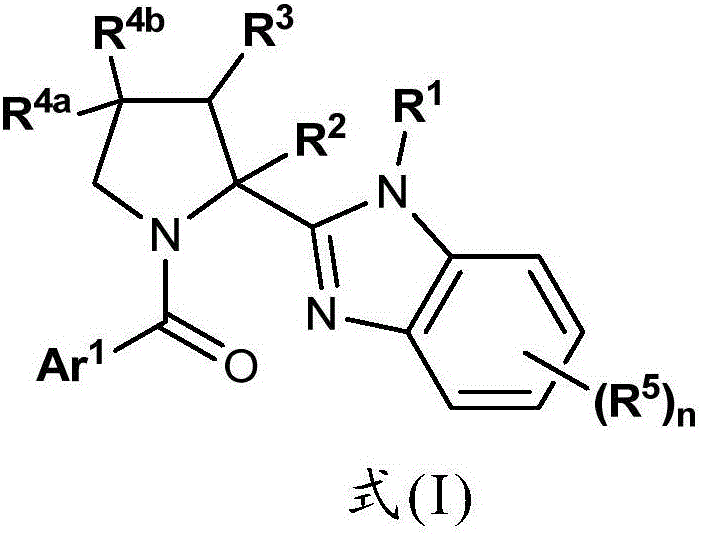
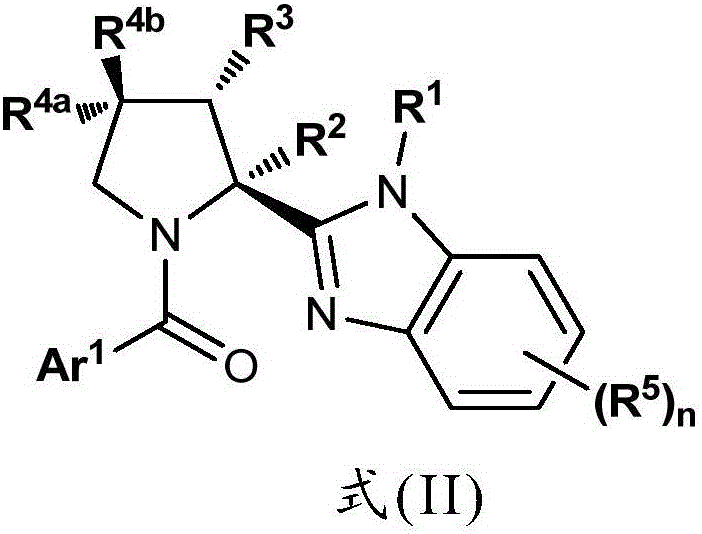
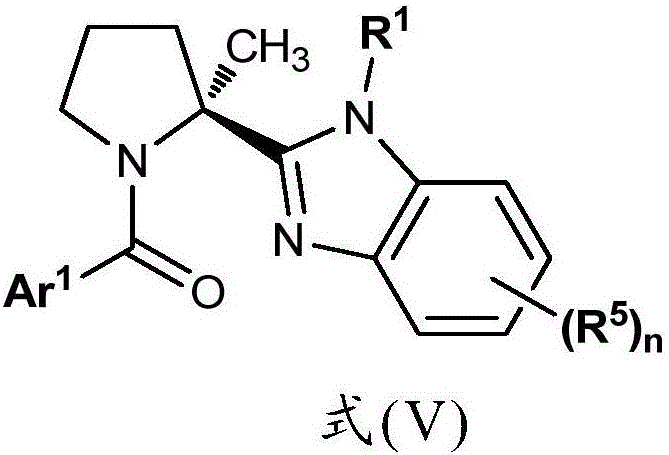
Benzimidazole Proline Derivatives
A benzimidazole and phenyl technology, applied in the field of benzimidazole proline derivatives, can solve problems such as no effective therapy yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1138] Preparative HPLC under acidic conditions
[1139] Method E: Column: Waters XBridge (10 μm, 75×30 mm). Conditions: MeCN [eluent A]; water + 0.5% HCOOH [eluent B]; gradient: 90% B→5% B over 6.4 minutes (flow rate: 75 mL / min). Detection: UV / Vis+MS.
[1140] Preparative HPLC under basic conditions
[1141] Method F: Column: Waters XBridge (10 μm, 75×30 mm). Conditions: MeCN [eluent A]; water + 0.5% NH 4 OH (25% in water) [eluent B]; Gradient: 90% B→5% B over 6.5 minutes (flow rate: 75 mL / min). Detection: UV / Vis+MS.
[1142] Abbreviations (as used in context):
[1143] Ac Acetyl (such as in OAc = acetate, AcOH = acetic acid)
[1144] AcOH acetic acid
[1145] anh. anhydrous
[1146] aq. Water-based
[1147] atm atmosphere
[1148] tBME tert-butyl methyl ether
[1149] Boc tert-butoxycarbonyl
[1150] Boc 2 O di-tert-butyl dicarbonate
[1151] BSA bovine serum albumin
[1152] Bu Butyl, such as in tBu = tert-butyl = tertiary butyl
[1153] CC silica gel column ...
Synthetic example 11
[1259]
[1260] Step 1: Mix 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid (2; 12.27g, 60.4mmol) and L-proline methyl ester hydrochloride ( 1; 10.20 g, 60.4 mmol) was dissolved in DCM (250 ml), followed by the addition of DIPEA (23.42 g, 31 ml, 181 mmol) and HATU (22.95 g, 60.4 mmol). Stirring was continued at room temperature for 1 hour, DCM was evaporated under reduced pressure and EtOAc (750ml) was added. The reaction mixture was extracted with brine (3 x 300ml). The organic layer was washed with MgSO 4 Dry, filter and evaporate the solvent under reduced pressure to give (S)-1-(5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoyl)pyrrolidine-2-carboxylic acid Methyl ester (3), which was used in the next step without further purification. LC-MS: t R = 0.66 minutes; [M+H] + = 315.09.
[1261] Step 2: Methyl (S)-1-(5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoyl)pyrrolidine-2-carboxylate (3, 18.81 g, 60.4 mmol) was dissolved in a mixture of MeOH (180 ml) and THF (210 ml), followed by...
Synthetic example 21
[1265]
[1266] Step 1: Dissolve (2S,4S)-1-(tert-butoxycarbonyl)-4-fluoropyrrolidine-2-carboxylic acid (7; 144.27mg, 0.6mmol) in a 1 / 1 mixture of DMF / DCM (1.2ml ), followed by the addition of DIPEA (0.44ml, 2.52mmol) and 4-chloro-3-methylbenzene-1,2-diamine (8; 121.95 mg, 0.6 mmol), and finally HATU (240 mg, 0.63 mmol) dissolved in DMF (1 ml) was added. The reaction mixture was stirred at room temperature for 15 hours, then passed through a PL-HCO-filled 1 / 1 mixture (4 ml) with DMF / DCM 3 filter syringe (1g). The solvent was removed under reduced pressure to afford tert-butyl (2S,4S)-2-((2-amino-4-chloro-3-methylphenyl)carbamoyl)-4-fluoropyrrolidine-1-carboxylate ( 9), which was used in the next step without further purification. LC-MS: t R = 0.79 minutes; [M+H] + = 372.26.
[1267] Step 2: (2S,4S)-2-((2-Amino-4-chloro-3-methylphenyl)carbamoyl)-4-fluoropyrrolidine-1-carboxylic acid tert-butyl ester (9; 220mg , 0.6 mmol) was dissolved in 100% AcOH (3 ml, 52.5 mmol) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com