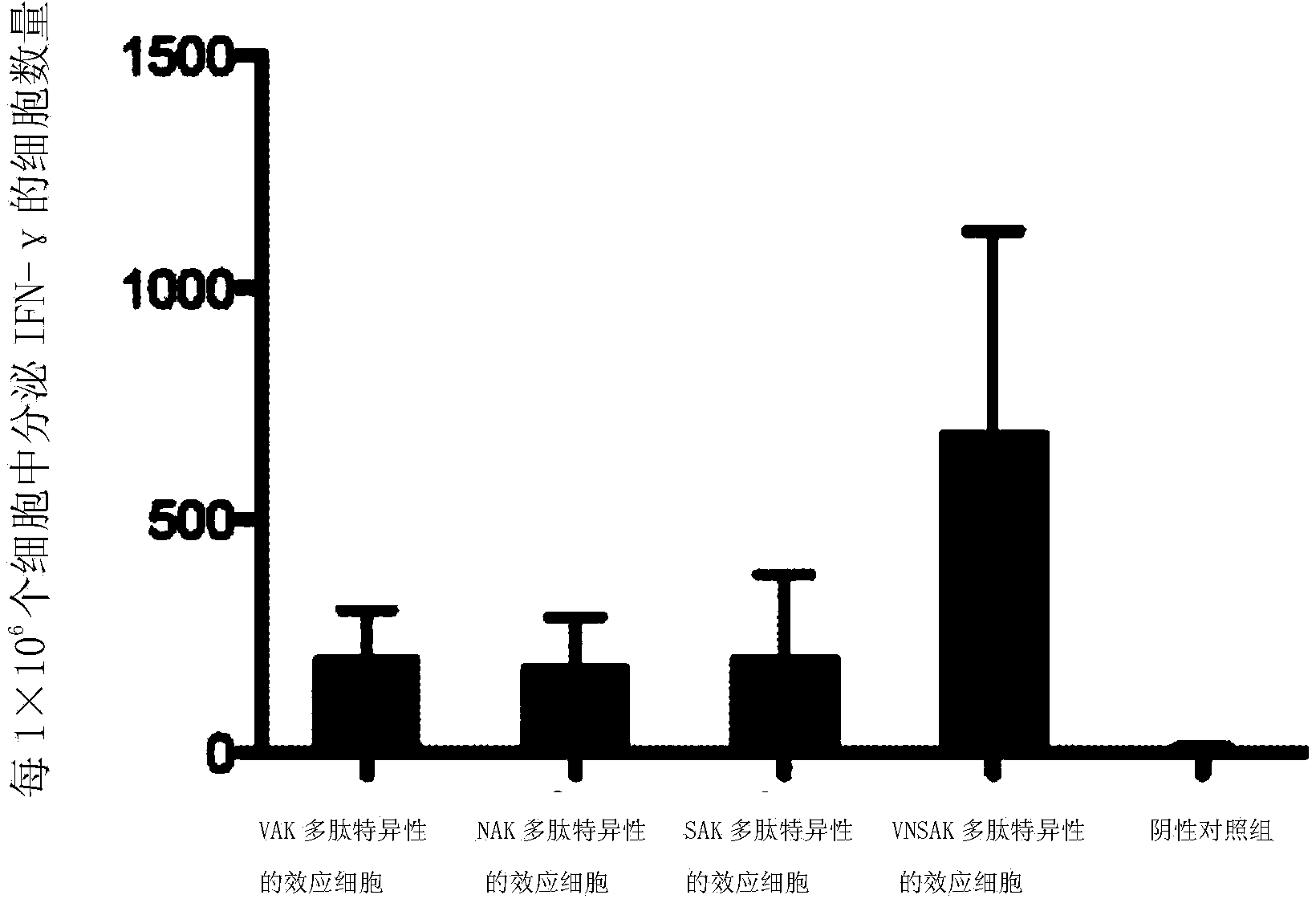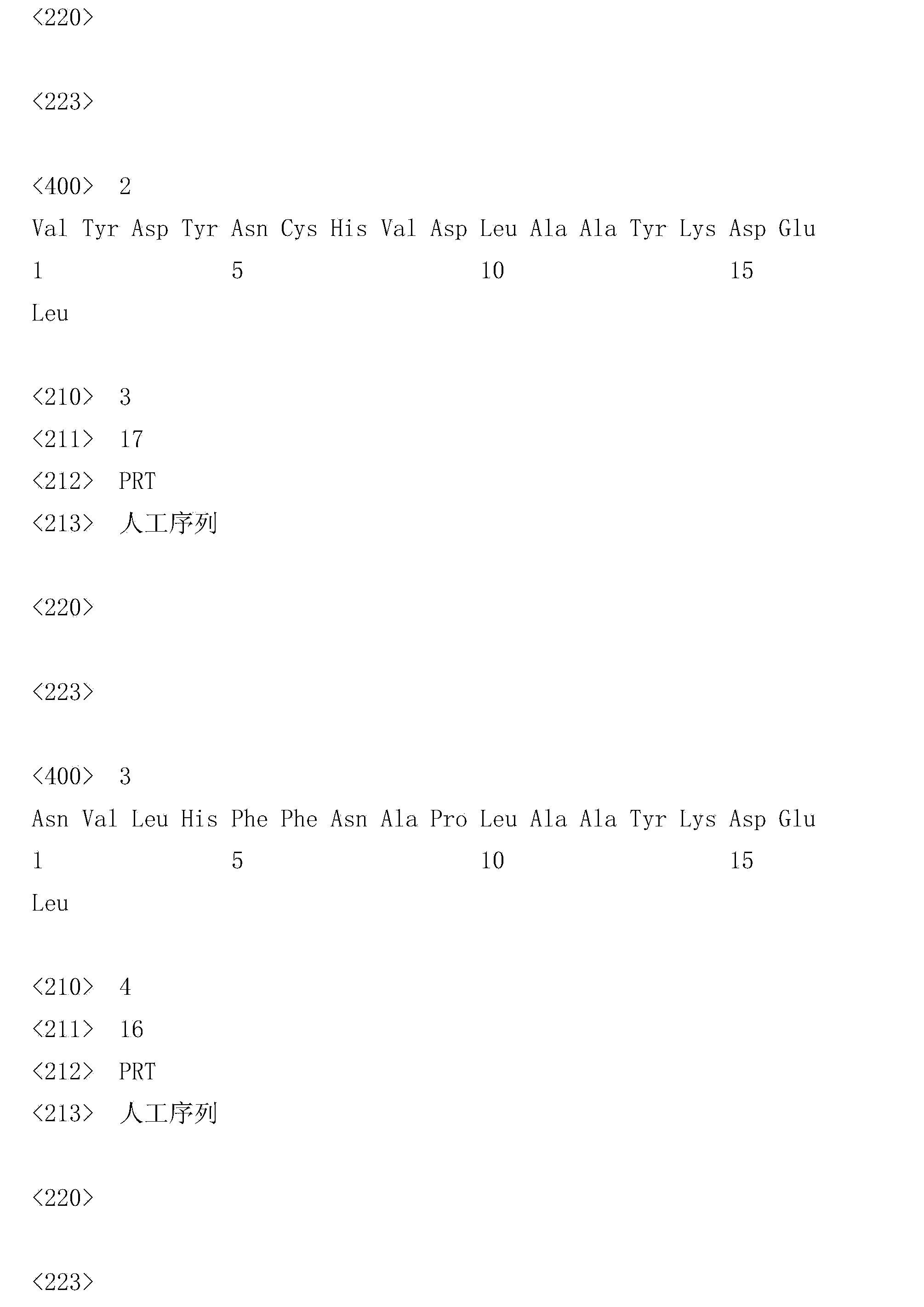VNSAK polypeptide and application thereof
An N-terminal and epitope technology, applied in the field of VNSAK polypeptides, can solve the problems of weak immunogenicity and immune tolerance, and achieve the effects of enhancing immunogenicity, overcoming weak immunogenicity, and overcoming immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of DC cells loaded with VNSAK polypeptide and effector cells specific for VNSAK polypeptide
[0032] 1. Preparation of DC cells loaded with VNSAK polypeptide
[0033] Principle: PBMC induces DC cells, DC cell phagocytosis, can swallow VNSAK polypeptide to form DC cells loaded with VNSAK polypeptide.
[0034] The cell culture in this step is performed at 37℃, 5% CO 2 In an incubator.
[0035] 1. Synthesize the polypeptide shown in sequence 1 of the sequence listing (named VNSAK polypeptide).
[0036] Sequence 1 of the sequence listing: VYDYNCHVDLNVLHFFNAPLSLLMWITQCAAYKDEL.
[0037] 2. Take human peripheral blood, isolate and obtain peripheral blood mononuclear cells (PBMC).
[0038] 3. Take the peripheral blood mononuclear cells obtained in step 2 and prepare 5×10 with RPMI-1640 medium 6 Cells / ml of cell suspension.
[0039] 4. Add the cell suspension obtained in step 3 to the cell culture flask and culture for 2 hours (to make DC cells adhere to the wall), the...
Embodiment 2
[0060] Example 2. The ability of effector cells to produce IFN-γ
[0061] ELISPOT detection kit (Human IFN-γELISPOT Kit): Shenzhen Juying Biotechnology Co., Ltd., item number "856 051 001", IFN-γ capture antibody, biotin-labeled anti-IFN-γ antibody and streptavidin label The alkaline phosphatase is a component of the kit. The effector cell suspension refers to the VNSAK-CTL system, VAK-CTL system, NAK-CTL system, or SAK-CTL system prepared in Example 1.
[0062] Take the effector cell suspension, use the ELISPOT detection kit and operate according to the kit instructions to detect the ability of various effector cells to produce IFN-γ. The specific steps are as follows: add 70% (volume ratio) ethanol aqueous solution to a 96-well plate at room temperature After 10 minutes of incubation, wash with PBS buffer, then add 100μl of IFN-γ capture antibody diluted to 100 times volume with PBS buffer to each well, incubate at 4°C for 12 hours, then wash with PBS buffer; then add effector c...
Embodiment 3
[0064] Example 3. The killing effect of effector cells on target cells
[0065] T2 cells: ATCC, ATCC number is " CRL-1992 TM ".
[0066] 1. Preparation of T2 cells loaded with antigen peptides
[0067] 1. Preparation of T2 cells loaded with VAK polypeptide
[0068] In RPMI-1640 culture medium, the artificially synthesized VAK polypeptide and T2 cells were heated at 37℃, 5% CO 2 Incubate for 24 hours under conditions (at the initial time of incubation, the concentration of VAK polypeptide is 50μg / ml, and the concentration of T2 cells is 1×1 0 6 cells / ml), and then collect the cells, which are T2 cells loaded with VAK polypeptide.
[0069] 2. Preparation of T2 cells loaded with NAK polypeptide
[0070] In RPMI-1640 culture medium, the artificially synthesized NAK polypeptide and T2 cells are heated at 37℃, 5% CO 2 Incubate for 24 hours under conditions (at the initial time of incubation, the concentration of NAK polypeptide is 50μg / ml, and the concentration of T2 cells is 1×10 6 Cells / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com