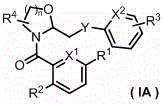
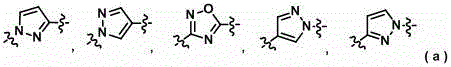
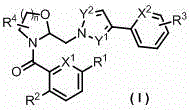
Heteroaromatic methyl cyclic amine derivatives
A methyl and alkyl technology, applied in the field of heteroaromatic methyl cyclic amine derivatives, can solve the problems of compounds without disclosed heteroaromatic methyl cyclic amine skeleton, compounds without disclosed methyl cyclic amine skeleton, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0454] Example 1: (-)-(2-{[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]methyl}-1,3-oxazolidin-3-yl )[5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
[0455] [Formula 44]
[0456]
[0457] Under ice-water cooling, [2-(hydroxymethyl)-1,3-oxazolidin-3-yl][5-methyl-2-(2H-1,2, 3-triazol-2-yl)phenyl]methanone (1.7 g, 5.9 mmol) and TEA (1.2 mL, 8.8 mmol) in CHCl 3 To a solution in (30 mL), MsCl (0.55 mL, 7.1 mmol) was added, and the resulting mixture was stirred for 1 hour. Under ice-water cooling, water was added to the reaction mixture, followed by CHCl 3 extraction. The organic layer was washed with saturated aqueous sodium chloride, washed with Na 2 SO 4 After drying, the desiccant was filtered off and the solvent was distilled off under reduced pressure. The obtained residue was purified by column chromatography (HP-Sil 50g, hexane / EtOAc=88 / 12 to 0 / 100) to obtain {3-[5-methyl-2-(2H-1,2,3- Triazol-2-yl)benzoyl]-1,3-oxazolidin-2-yl}methylsulfonate (pale yellow oil). 5...
Embodiment 5
[0464] Example 5: (-)-[(2S,5S)-2-{[4-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]methyl}-5-methyl- 1,3-oxazolidin-3-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
[0465] [Formula 45]
[0466]
[0467] By using (2RS,5S)-5-methyl-3-[5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoyl] obtained in Reference Example 5 -1,3-Oxazolidine-2-carboxylic acid ethyl ester (0.11g, 0.33mmol) as starting material, the same procedure as in Reference Example 2 was carried out to obtain [2-(hydroxymethyl)-5-methyl-1 ,3-oxazolidin-3-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone diastereomeric mixture (without color oil). The diastereomeric mixture obtained was purified by thin layer chromatography (1 mm, hexane / EtOAc=50 / 50) to obtain [(2S,5S)-2-(hydroxymethyl)-5-methyl-1 ,3-oxazolidin-3-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (colorless oil). Under ice-water cooling, to the obtained [(2S,5S)-2-(hydroxymethyl)-5-methyl-1,3-oxazolidin-3-yl][5-methyl-2-( 2H-1,2,3-triazol...
Embodiment 11
[0474] Example 11: (±)-(2-{[3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]methyl}-1,3-oxazolidin-3-yl )[2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
[0475] [Formula 46]
[0476]
[0477] To 2-[1-(2,2-diethoxyethyl)-1H-pyrazol-3-yl]-5-fluoropyridine (4.0 g, 14.3 mmol) obtained in Reference Example 12 in CHCl 3 To a solution in (72 mL), TFA (6.4 mL, 85.9 mmol) was added, and the resulting mixture was stirred at 35°C for 6 hours. TFA (6.4 mL, 85.9 mmol) was added thereto, and the mixture was stirred at 35°C for 3 hours. The reaction mixture was cooled to room temperature, then NaHCO 3 Aqueous solution was added to the reaction mixture, followed by CHCl 3 extraction. The organic layer was washed with saturated aqueous sodium chloride, washed over MgSO 4 Dry, then filter off the desiccant. The solvent was distilled off under reduced pressure to obtain [3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]acetaldehyde (colorless oil). To [3-(5-fluoropyridin-2-yl)-1H-pyrazol-1-yl]acetaldehy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com