Applications of protopanaxatriol and derivatives thereof in preparation of medicines for treating hepatic disease
A technology of protopanaxatriol and derivatives, which is applied in the field of medicine, can solve the problems of few reaction sites, difficult to prepare derivatives, lack of active groups, etc., and achieves the effect of wide use and good anti-liver fibrosis activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, preparation of protopanaxatriol derivatives of the present invention
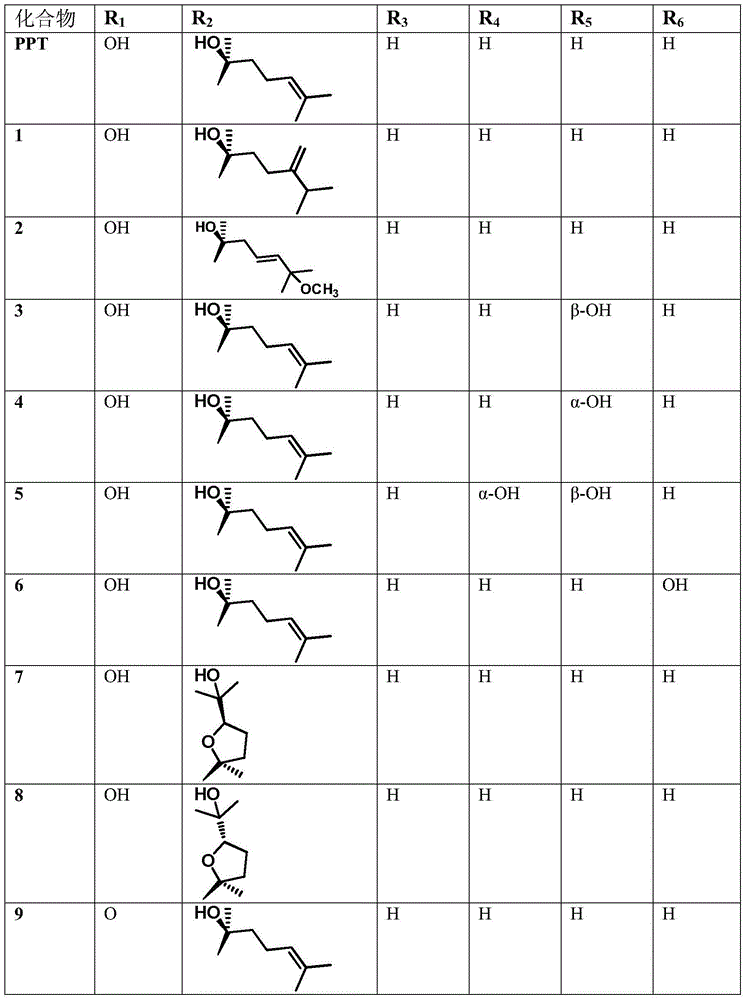
[0022] References Yin Tian, Hongzhu Guo, Jian Han, Dean Guo. Microbial Transformation of 20(S)-Protopanaxatriol by Mucor spinosus. Journal of Natural Products 2005, 68, 678-680; Jie Zhang, Hongzhu Guo, Yin Tian, Peng Liu, Na Li, Jianping Zhou, Dean Guo. Biotransformation of 20(S)-protopanaxatriol by Mucor spinosus and the cytotoxic structure activity relationships of the transformed products. Phytochemistry 2007, 69, 2523-2530 and Guangtong Chen, Xue Yang, Xuguang Zhai, Min Yang .Microbial transformation of 20(S)-protopanaxatriol by Absidia corymbifera and their cytotoxic activities against two human prostate cancer cell lines.Biotechnology Letters 2013,35,91-95 prepared compounds 2-15, 19 and 20 using microbial transformation methods .
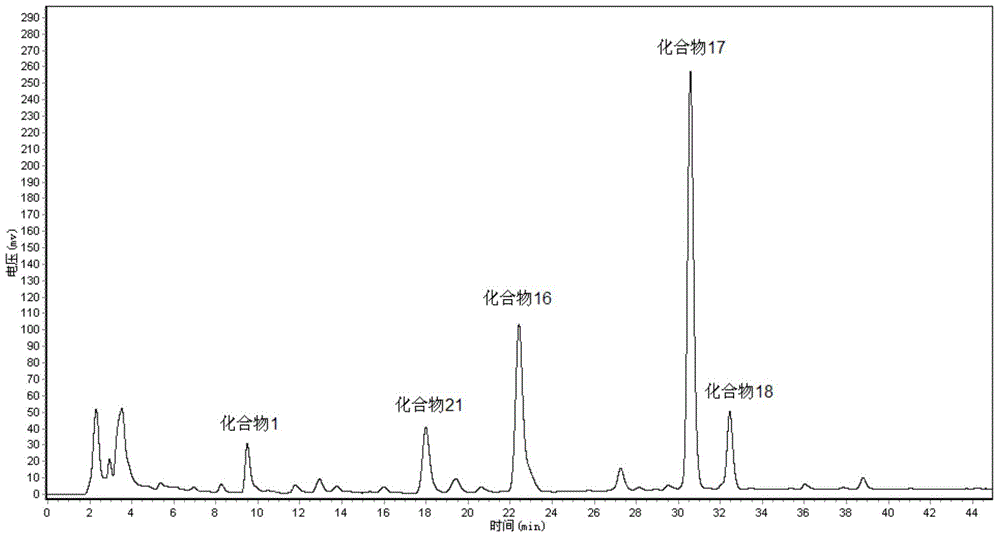
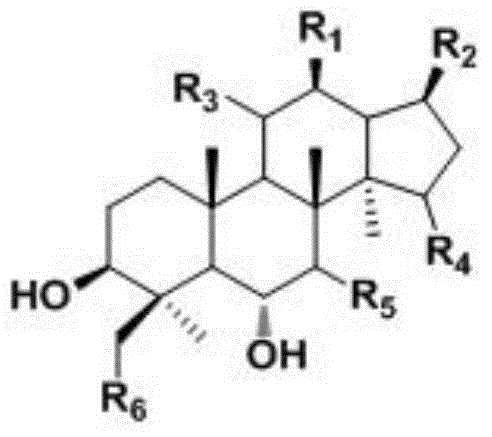
[0023] Protopanaxatriol derivatives of the present invention, compounds 1,21,16,17,18 are prepared as follows:
[0024] The invention adopts a m...
Embodiment 2
[0055] Example 2 Anti-hepatic fibrosis activity of protopanaxatriol (PPT) and protopanaxatriol derivatives (compound 1-21) according to the present invention
[0056] 1) Experimental materials
[0057] Instruments and reagents: CO 2 Incubator (Jouan IGO150); Microplate reader (Bio-TEK ELx800); Fluorescence inverted microscope (Olympus IX51); MTT cell proliferation and cytotoxicity detection kit (Biotech Institute), RPM I1640 medium (Gibcol BRL ), RNase A, fetal bovine serum, dimethyl sulfoxide (DMSO), trypsin (Shanghai Bioengineering Co., Ltd.).
[0058] Cell line for testing: HSC-T6 cell line, which is SD rat hepatic stellate cells transfected with SV40, was purchased from Cancer Institute, Chinese Academy of Medical Sciences.
[0059] Test samples: protopanaxatriol (PPT) and compound 1-21 synthesized in Example 1, with a purity of over 90%, each compound was dissolved in DMSO and then diluted.
[0060] 2) Experimental method
[0061] The half inhibitory rate IC of each t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com