A kind of method of olefin epoxidation
An epoxidation and epoxidation reaction technology, applied in chemical instruments and methods, catalytic reactions, organic chemistry, etc., can solve the problems of low reaction efficiency, corroded equipment, low selectivity, etc., and achieve high catalytic efficiency and improved selection. The effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: styrene epoxidation reaction
[0048] In a round bottom flask with magnetic stirring, add 1 mmol of styrene, 0.015 mmol of methyl rhenium trioxide, 0.6 mL of 1-butyl-3-methyl-imidazole perrhenate ([BMIM][ReO 4 ] ionic) liquid, after adding 1.5mmol oxidizing agent carbamide peroxide, the reaction was started. Stir magnetically for 8 hours at room temperature. After the reaction, the solubility of styrene and 1,2 styrene oxide in ether is higher than that in [BMIM][ReO 4 ]Characteristics of ionic liquids, injection after multiple extractions with ether.
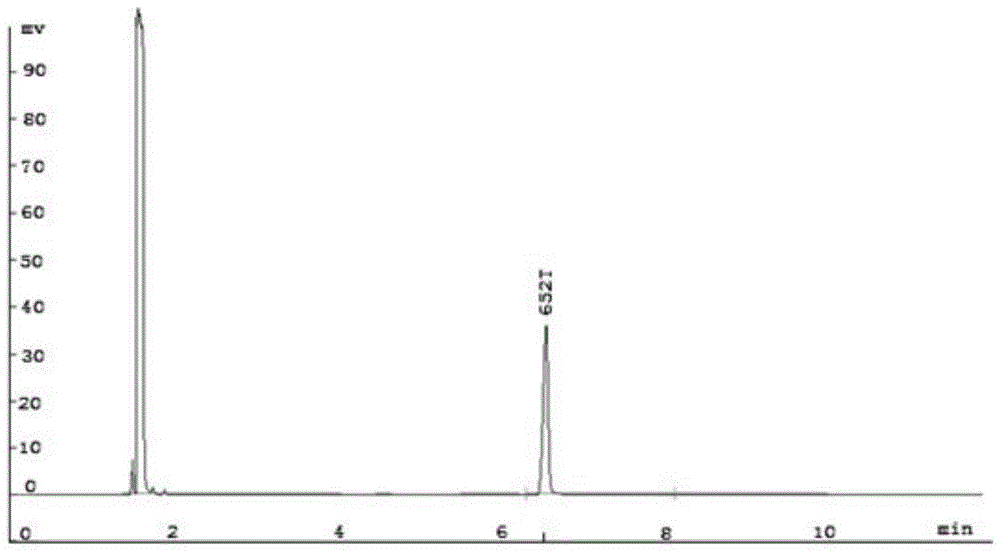
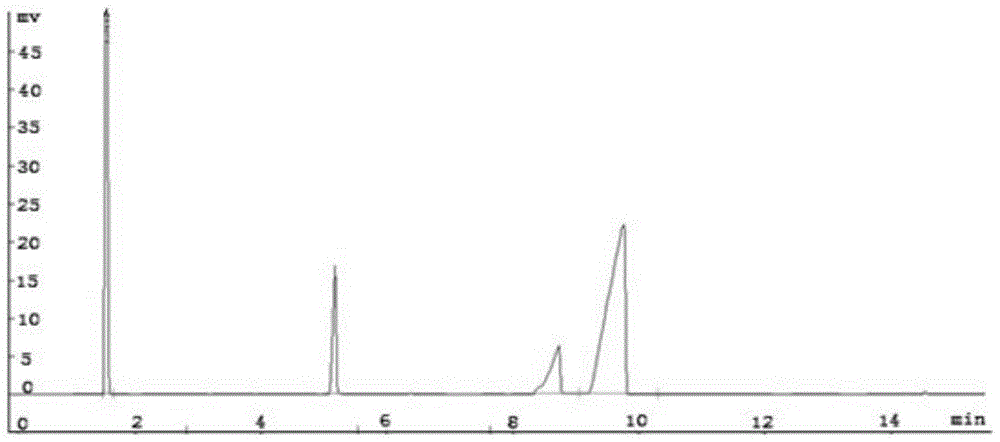
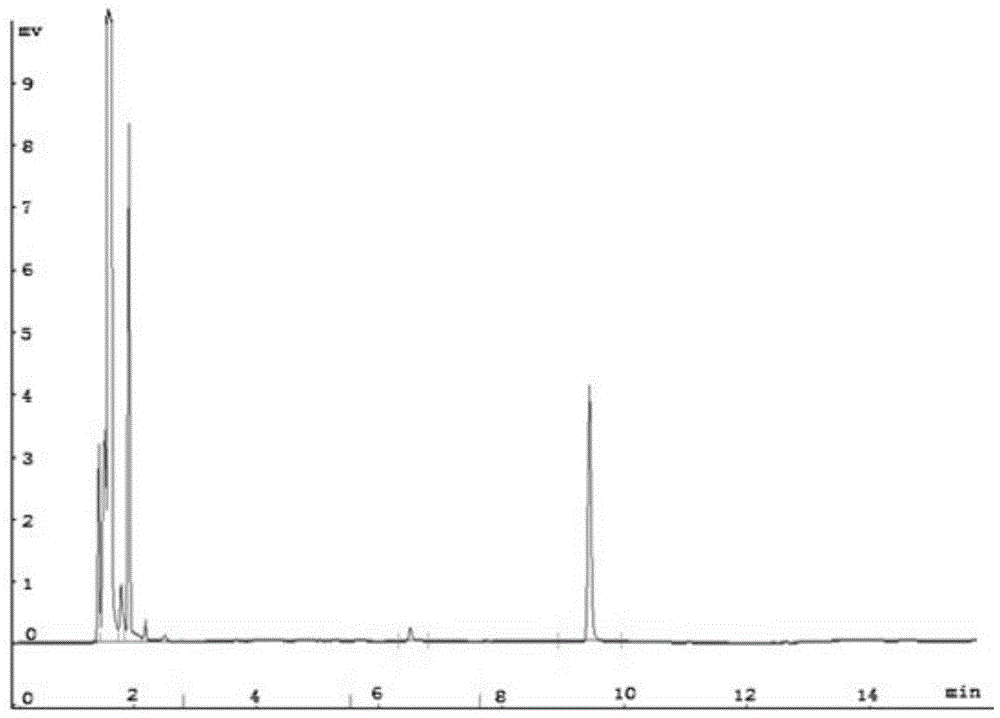
[0049] Detected by gas chromatography, the results are as follows Figure 3-5 As shown in the figure, the peak at 6.490min in retention time is the peak of raw material styrene, and the peak at 9.418min in retention time is the peak of 1,2 styrene oxide. It can be seen from the figure that the reaction generates a single product, and the yield was 94.7%.
Embodiment 2
[0050] Embodiment 2: styrene epoxidation reaction
[0051] In a round bottom flask with magnetic stirring, add 1 mmol of styrene, 0.01 mmol of methyl rhenium trioxide, 0.7 mL of [BMIM][ReO 4 ] ionic liquid, after adding 2.5mmol oxidizing agent carbamide peroxide, the reaction was started. Stir magnetically for 8 hours at room temperature. After the reaction, the solubility of styrene and 1,2 styrene oxide in ether is higher than that in [BMIM][ReO 4 ]Characteristics of ionic liquids, injection after multiple extractions with ether.
[0052] Detected by gas chromatography, the peak whose retention time is located at 6.490min is the peak of raw material styrene, and the peak whose retention time is located at 9.418min is the peak of 1,2 styrene oxide. It can be seen from the figure that the reaction generates a single product, and the yield is 90.6%.
Embodiment 3
[0053] Embodiment 3: styrene epoxidation reaction
[0054] In a round bottom flask with magnetic stirring, add 1 mmol of styrene, 0.05 mmol of methyl rhenium trioxide, 0.7 mL of 1-pentyl-3-methyl-imidazole perrhenate ([PMIM][ReO 4 ] ionic) liquid, after adding 2.5mmol oxidizing agent carbamide peroxide, the reaction was started. Stir magnetically for 8 hours at room temperature. After the reaction, the solubility of styrene and 1,2 styrene oxide in ether is higher than that of [PMIM][ReO 4 ]Characteristics of ionic liquids, injection after multiple extractions with ether.
[0055] Detected by gas chromatography, the peak whose retention time is located at 6.490min is the peak of raw material styrene, and the peak whose retention time is located at 9.418min is the peak of 1,2 styrene oxide. It can be seen from the figure that the reaction generates a single product, and the yield is 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com