A method for preparing high-purity magnesium oxide by utilizing magnesium carbonate coarse ore
A technology of coarse magnesium carbonate ore and magnesium oxide, applied in magnesium oxide and other directions, can solve the problems of high production cost, large resin consumption, inability to remove boron compound impurities, etc., and achieves the effects of convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
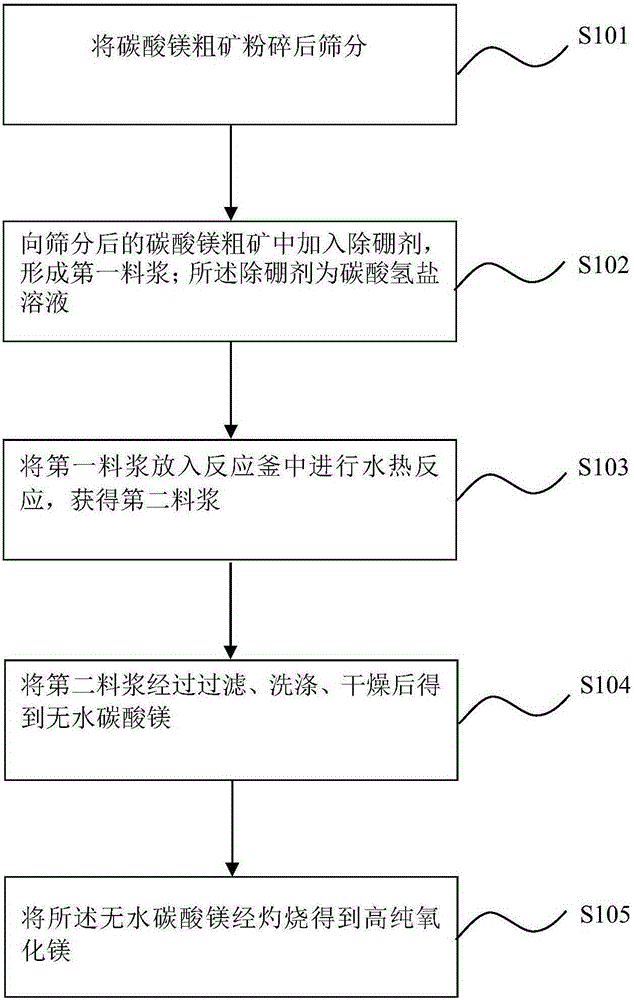
[0033] 1. The raw material is magnesium carbonate coarse ore, which is the primary product of Longmu Co and Jiezechaka salt lake brine (sulphate type and carbonate type salt lake brine), and the main component of the raw material is 67.2% 4MgCO 3 ·Mg(OH) 2 ·5H 2 O, the secondary components are NaCl and a small amount of sulfate and 0.27% of boron-containing compounds (calculated as B), etc. First use 80 mesh screens to sieve after magnesium carbonate coarse ore is pulverized, adopt the method for traditional washing, filtration to remove soluble impurity (for example use deionized water to be made into slurry according to the liquid-solid ratio of 5:1 by weight, Filtration in vacuum after mechanical stirring, repeated twice).
[0034] Two, adding mass concentration to the magnesium carbonate coarse ore after screening and removing soluble impurities is 3% sodium bicarbonate solution as boron removal agent, wherein, according to mass ratio calculation, the ratio of sodium bic...
Embodiment 2
[0040] 1. The raw material is magnesium carbonate coarse ore, which is the primary product of Longmu Co and Jiezechaka salt lake brine (sulphate type and carbonate type salt lake brine), and the main component of the raw material is 67.2% 4MgCO 3 ·Mg(OH) 2 ·5H 2O, the secondary components are NaCl and a small amount of sulfate and 0.27% of boron-containing compounds (calculated as B), etc. First use 60 mesh sieves to sieve after magnesium carbonate coarse ore is pulverized, adopt traditional washing, the method for filtering to remove soluble impurity (for example use deionized water to be made into slurry according to the liquid-solid ratio of 5:1 by weight, Filtration in vacuum after mechanical stirring, repeated twice).
[0041] Two, adding mass concentration to the magnesium carbonate coarse ore after screening and removing the soluble impurities is 9% sodium bicarbonate solution as boron removal agent, wherein, calculated according to mass ratio, the ratio of sodium bic...
Embodiment 3
[0047] 1. The raw material is magnesium carbonate coarse ore, which is the primary product of Longmu Co and Jiezechaka salt lake brine (sulphate type and carbonate type salt lake brine), and the main component of the raw material is 67.2% 4MgCO 3 ·Mg(OH) 2 ·5H 2 O, the secondary components are NaCl and a small amount of sulfate and 0.27% of boron-containing compounds (calculated as B), etc. First use 80 mesh screens to sieve after magnesium carbonate coarse ore is pulverized, adopt the method for traditional washing, filtration to remove soluble impurity (for example use deionized water to be made into slurry according to the liquid-solid ratio of 5:1 by weight, Filtration in vacuum after mechanical stirring, repeated twice).
[0048] Two, adding mass concentration to the magnesium carbonate coarse ore after screening and removing soluble impurities is 6% sodium bicarbonate solution as boron removal agent, wherein, according to mass ratio calculation, the ratio of sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com