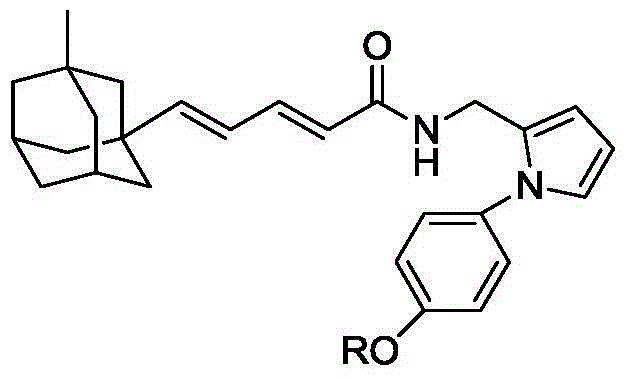
Compound containing alcoxyl phenyl group and diene adamantane structure and preparation method and application thereof
A compound and alkyl technology, applied in the field of drugs related to thrombosis diseases, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 2.43g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20 mL of dry THF and reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white cloudy solution was obtained. Cooled to -78°C under nitrogen protection, slowly added 6.25mL (10mmol, 1.6M) n-BuLi n-hexane solution dropwise. After the dropwise addition was completed, stirring was continued for one hour, and a solution prepared by dissolving compound 1.28g (10mmol) III in 2mL of THF was added dropwise. After the dropwise addition was complete, the reaction mixture was slowly warmed to room temperature and then refluxed for 1 hour. The reaction mixture was poured into ice water, stirred, extracted with 50 mL×3 dichloromethane, the combined extracts were washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, 0.50 g of iodine was added to the filtrate, and stirred overnight at room temperature....
Embodiment 2-5
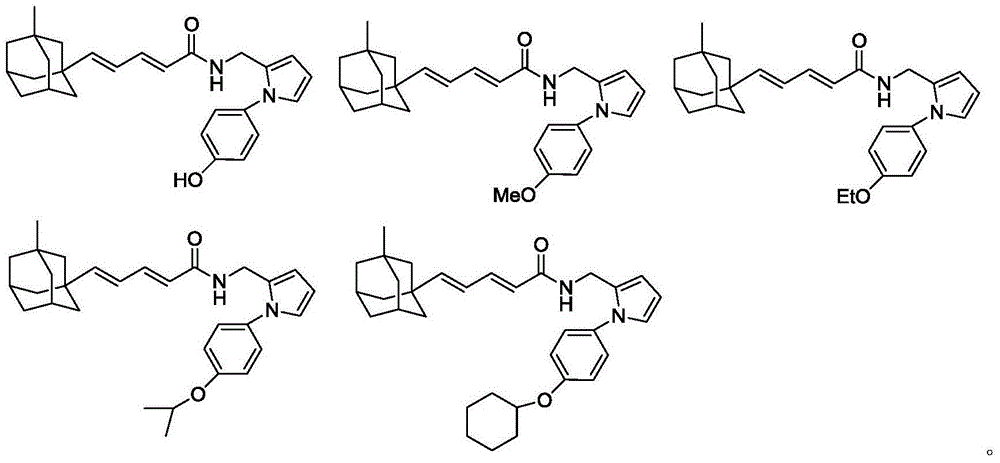
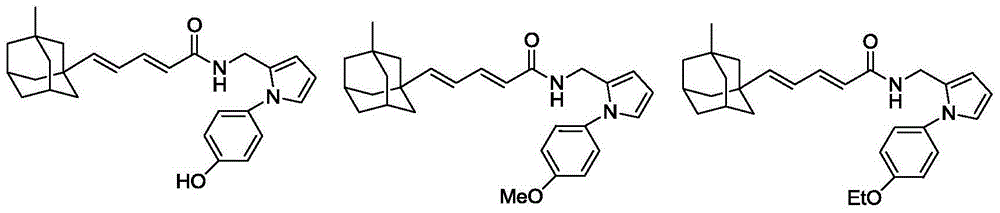
[0032] Referring to the method of Example 1, the following compounds with general formula I were synthesized.
[0033]
[0034]
Embodiment 6
[0035] The preparation of embodiment 6 reference compound D1
[0036] In order to fully illustrate the beneficial effects of the compounds of the present invention, the applicant recorded the following compound D1 (unpublished) found during the experiment as a pharmacodynamic reference compound.
[0037]
[0038] The synthesis method is as follows:
[0039]
[0040] 2.43g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20 mL of dry THF and reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white cloudy solution was obtained. Cooled to -78°C under nitrogen protection, slowly added 6.25mL (10mmol, 1.6M) n-BuLi n-hexane solution dropwise. After the dropwise addition was completed, stirring was continued for one hour, and a solution prepared by dissolving compound 1.28g (10mmol) III in 2mL of THF was added dropwise. After the addition was complete, the reaction mixture was slowly warmed to room temperature a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com