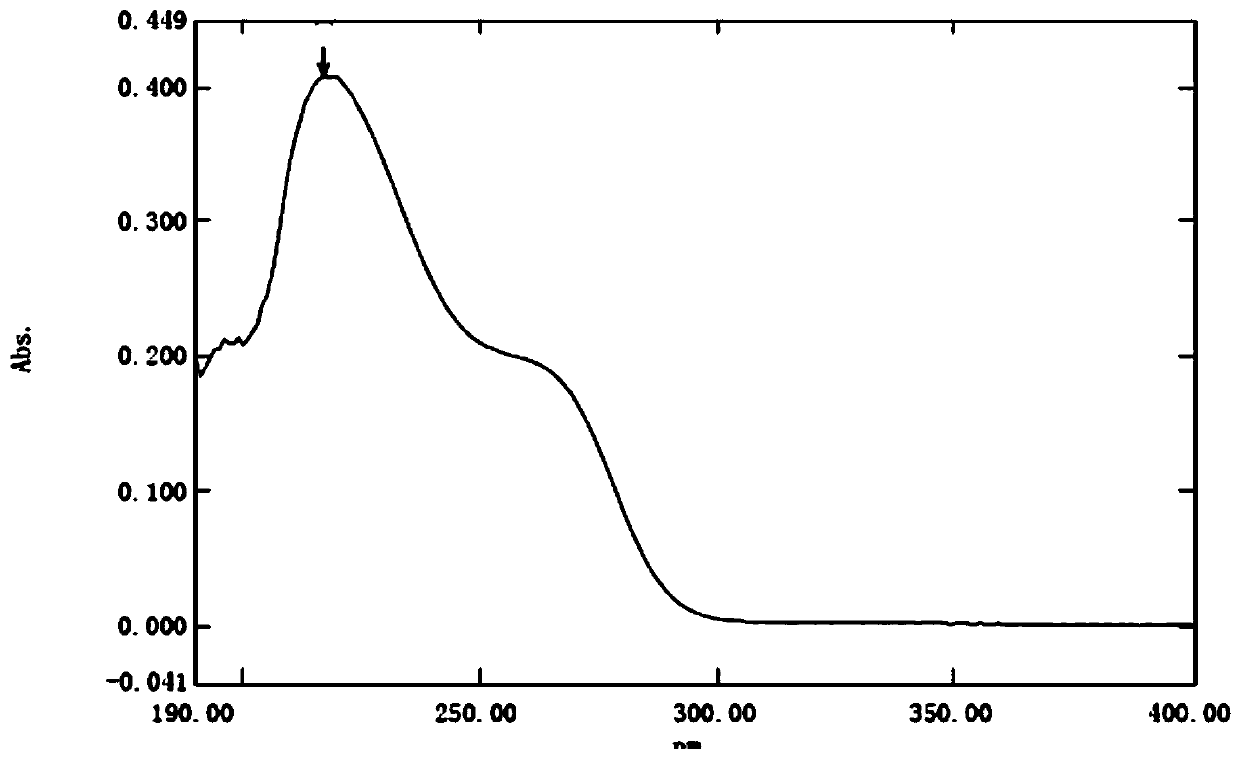
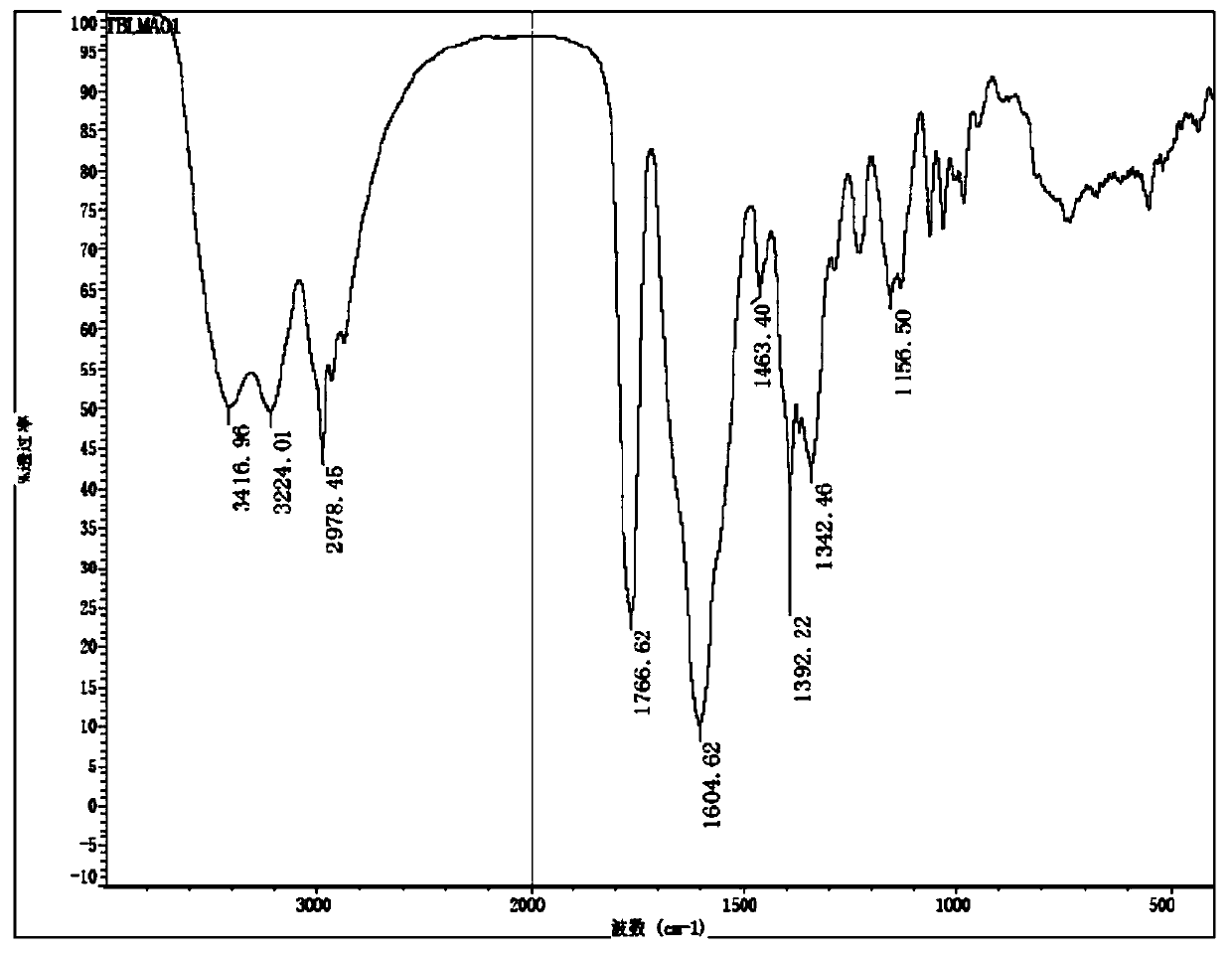
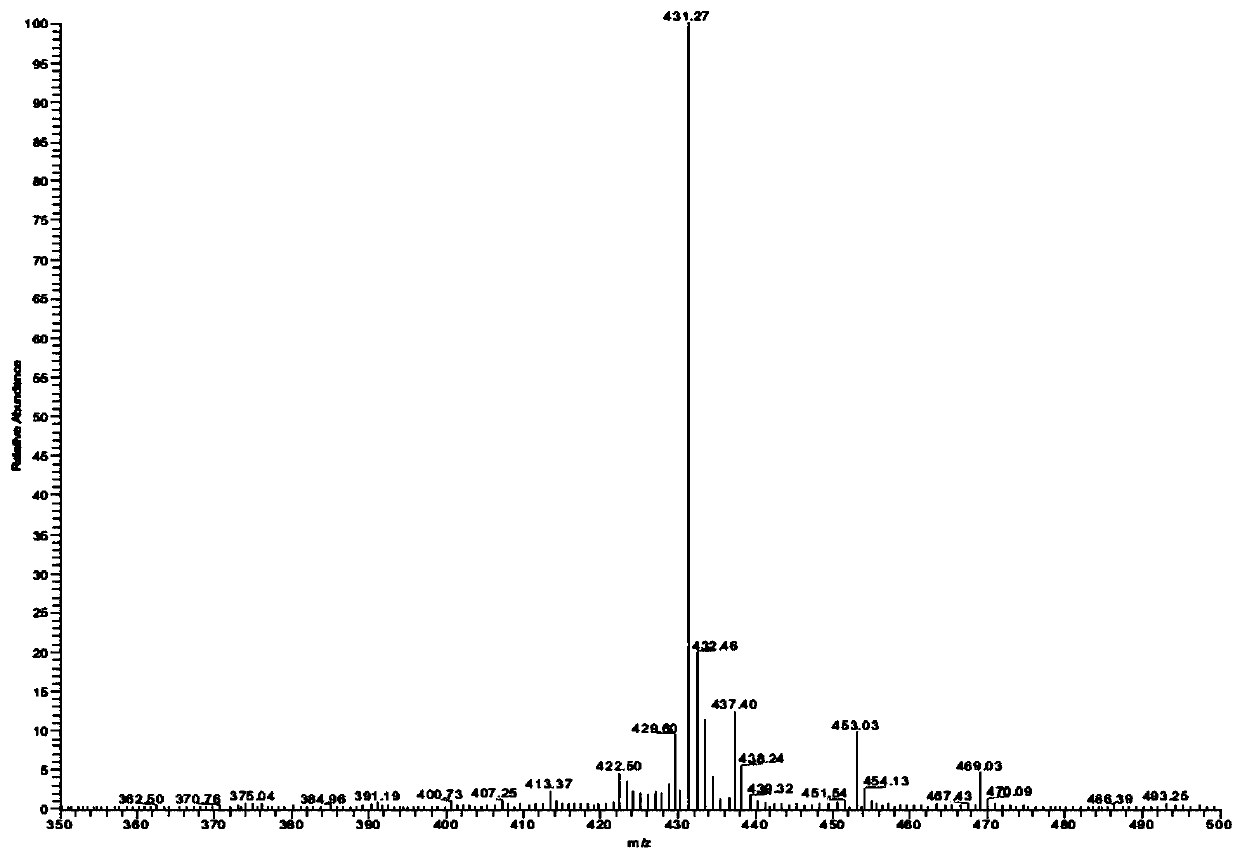
A kind of preparation method of deacetylceftiamidine
A technology of acetylceftiamidine and cefathiamidine, which is applied in the field of medicine, can solve problems such as inability to confirm the structure, and achieve the effects of low cost, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 20g of cefathiamidine, add 50ml of purified water, control the temperature at 20°C to 25°C, stir until it dissolves, add 1.6g of immobilized acetylesterase, and continuously and slowly add triethylamine to control the pH to 7.0 to 9.0, and stir to react until The pH remained essentially unchanged. Filter to remove the enzyme, add hydrochloric acid dropwise to adjust the pH to 5.0-5.5, concentrate the filtrate to dryness under reduced pressure, wash with dichloromethane, filter, and dry in vacuo to obtain deacetylceftiamidine.
[0026] The purity was 98% as detected by HPLC.
Embodiment 2
[0028] Add 20g of cefathiamidine, 60ml of purified water and 3ml of methanol, control the temperature at 20°C-25°C, stir until it dissolves, add 2.0g of immobilized acetylesterase, and continuously and slowly add ammonia water dropwise to control the pH 7.0-9.0, and stir to react until the pH remained basically unchanged. Filter to remove the enzyme, add hydrochloric acid dropwise to adjust the pH to 5.0-5.5, concentrate the filtrate to dryness under reduced pressure, wash with acetone, filter, and vacuum-dry to obtain deacetylceftiamidine.
[0029] The purity was 97% as detected by HPLC.
Embodiment 3
[0031] Add 20g of cefathiamidine, 70ml of purified water and 7ml of ethanol, control the temperature at 20°C-25°C, stir until it dissolves, add 2.2g of immobilized acetylesterase, and continuously and slowly drop triethylamine to control the pH 7.0-9.0, The reaction was stirred until the pH remained essentially constant. Filter to remove the enzyme, add hydrochloric acid dropwise to adjust the pH to 5.0-5.5, concentrate the filtrate to dryness under reduced pressure, wash with dichloromethane, filter, and dry in vacuo to obtain deacetylceftiamidine.
[0032] The purity was 97% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com