Method for preparing chloroacetic acid by using intermittent chlorination process
A technology of chloroacetic acid and chlorosulfonic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as long reaction time and high content of dichlorine, shorten reaction time, reduce production, and improve reaction efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
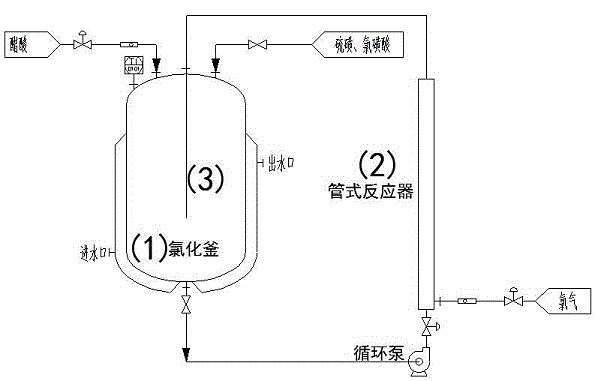
[0013] Embodiment 1, a kind of method for producing monochloroacetic acid by intermittent chlorination, its steps are: load weighed acetic acid in the chlorination reactor, then add the main catalyst sulfur whose quality is 1% of the quality of acetic acid successively, the mass The cocatalyst chlorosulfonic acid is 0.5% of the mass of acetic acid, and then the reaction kettle is heated to 70°C, and the circulation pump is started to allow the reaction liquid and chlorine gas in the kettle to enter from the lower end of the 2.5-meter tubular reactor for chlorination reaction. The upper end of the reactor is returned to the reactor, and the reaction time is 0.5 hour. Then, the temperature of the reactor is raised to 100° C., and the reaction liquid and chlorine continue to circulate through the tubular reactor for 6.0 hours to the end.
[0014] After the reaction, the content of monochloroacetic acid was 95.0% and the content of dichloroacetic acid was 1.2% in the product liquid...
Embodiment 2
[0015] Embodiment 2, a kind of method for producing monochloroacetic acid by intermittent chlorination, its steps are: load weighed acetic acid in the chlorination reactor, then add the main catalyst sulfur whose quality is 4% of the acetic acid quality, the mass The cocatalyst chlorosulfonic acid is 0.2% of the mass of acetic acid, and then the reaction kettle is heated to 75°C, and the circulation pump is started to allow the reaction liquid and chlorine gas in the kettle to enter from the lower end of the 3.0-meter tubular reactor for chlorination reaction. The upper end of the reactor is returned to the reactor, and the reaction time is 1.0 hour. Then, the temperature of the reactor is raised to 95° C., and the reaction liquid and chlorine continue to circulate through the tubular reactor for 8.0 hours to the end.
[0016] After the reaction, the content of monochloroacetic acid in the product liquid was 94.8%, and the content of dichloroacetic acid was 1.0%.
Embodiment 3
[0017] Embodiment 3, a kind of method for producing monochloroacetic acid by intermittent chlorination, its steps are: load weighed acetic acid in the chlorination reactor, then add the main catalyst sulfur whose quality is 2.5% of the acetic acid quality, the mass 0.35% chlorosulfonic acid as a co-catalyst of acetic acid quality, then the reaction kettle is heated to 73 ° C, and the circulation pump is started to allow the reaction liquid and chlorine in the kettle to enter from the lower end of the 2.8-meter tubular reactor for chlorination reaction, and then from the The upper end of the tubular reactor was returned to the reactor, and the reaction time was 0.8 hours. Then, the temperature of the reactor was raised to 98° C., and the reaction liquid and chlorine continued to circulate through the tubular reactor for 7.0 hours to the end.
[0018] After the reaction finished, the content of monochloroacetic acid was 95.2% and the content of dichloroacetic acid was 1.1% in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com