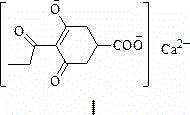
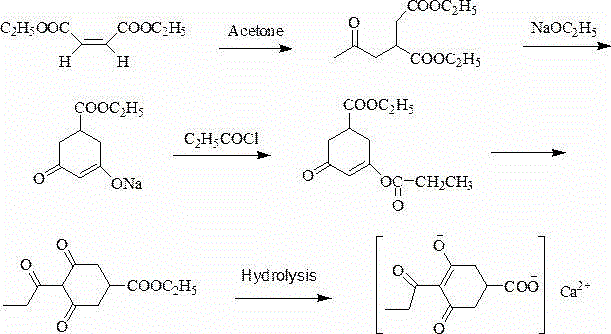
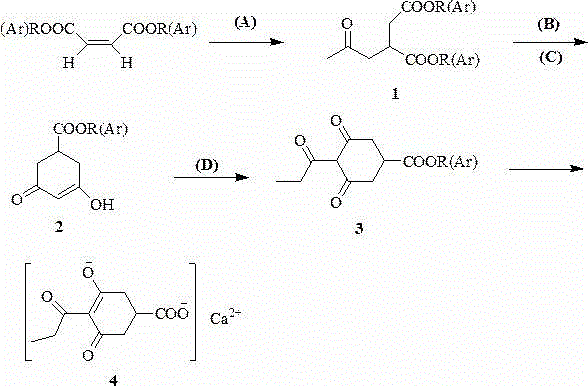
Preparation method of prohexadione calcium
A technology of prohexadione and dioxocyclohexanecarboxylate, which is applied in the field of preparation of prohexadione calcium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Place dioctyl maleate (680.00 g, 2.00 mol), acetone (84.00 g, 4.00 mol) and diethylamine (14.60 g, 0.20 mol) in an autoclave and react at 150 ℃ for 24 h. The solvent was spin-dried under reduced pressure to obtain product 1: Diisooctyl acetonyl succinate. Transfer diisooctyl acetonyl succinate to a three-necked round bottom flask, add dropwise the ethanol solution of sodium ethoxide (1 mol / L, 2.00 mol) with mechanical stirring and control the reaction temperature below 5 ℃. After the dripping is completed, continue to stir at 5 ℃ for 2 h, then rise to room temperature and stir for 12 h; after the solution is cooled below 5 ℃, add a quantitative amount of glacial acetic acid to neutralize the reaction solution, and recover ethanol under reduced pressure to obtain the product 2:3 , 5-Dioxocyclohexanecarboxylic acid isooctyl ester. Add 1,2-dichloroethane (2.7 L) into the reaction vessel containing isooctyl 3,5-dioxocyclohexane carboxylate, and add propionyl chloride (202...
Embodiment 2
[0041] Place dioctyl maleate (680.00 g, 2.00 mol), acetone (84.00 g, 4.00 mol) and diethylamine (14.60 g, 0.20 mol) in an autoclave and react at 150 ℃ for 24 h. The solvent was spin-dried under reduced pressure to obtain product 1: Diisooctyl acetonyl succinate. Transfer diisooctyl acetonyl succinate to a three-necked round bottom flask, add dropwise the ethanol solution of sodium ethoxide (1 mol / L, 2.00 mol) with mechanical stirring and control the reaction temperature below 5 ℃. After the addition is complete, continue to stir at 5 ℃ for 2 h, then warm to room temperature and stir for 12 h; after the solution is cooled below 5 ℃, add a quantitative amount of methanesulfonic acid to neutralize the reaction solution, and recover ethanol under reduced pressure to obtain product 2 :3,5-Dioxocyclohexanecarboxylic acid isooctyl ester. Add 1,2-dichloroethane (2.7 L) into the reaction vessel containing isooctyl 3,5-dioxocyclohexane carboxylate, and add propionyl chloride (202.40 g...
Embodiment 3
[0043] Place dioctyl maleate (680.00 g, 2.00 mol), acetone (84.00 g, 4.00 mol) and diethylamine (14.60 g, 0.20 mol) in an autoclave and react at 150 ℃ for 24 h. The solvent was spin-dried under reduced pressure to obtain product 1: Diisooctyl acetonyl succinate. Transfer diisooctyl acetonyl succinate to a three-necked round bottom flask, add dropwise the ethanol solution of sodium ethoxide (1 mol / L, 2.00 mol) with mechanical stirring and control the reaction temperature below 5 ℃. After the dripping is completed, continue to stir at 5 ℃ for 2 h, then warm to room temperature and stir for 12 h; after the solution is cooled below 5 ℃, add a quantitative amount of phosphoric acid to neutralize the reaction solution, and recover ethanol under reduced pressure to obtain the product 2:3, Isooctyl 5-dioxocyclohexane carboxylate. Add 1,2-dichloroethane (2.7 L) into the reaction vessel containing isooctyl 3,5-dioxocyclohexane carboxylate, and add propionyl chloride (202.40 g, 2.20 mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com