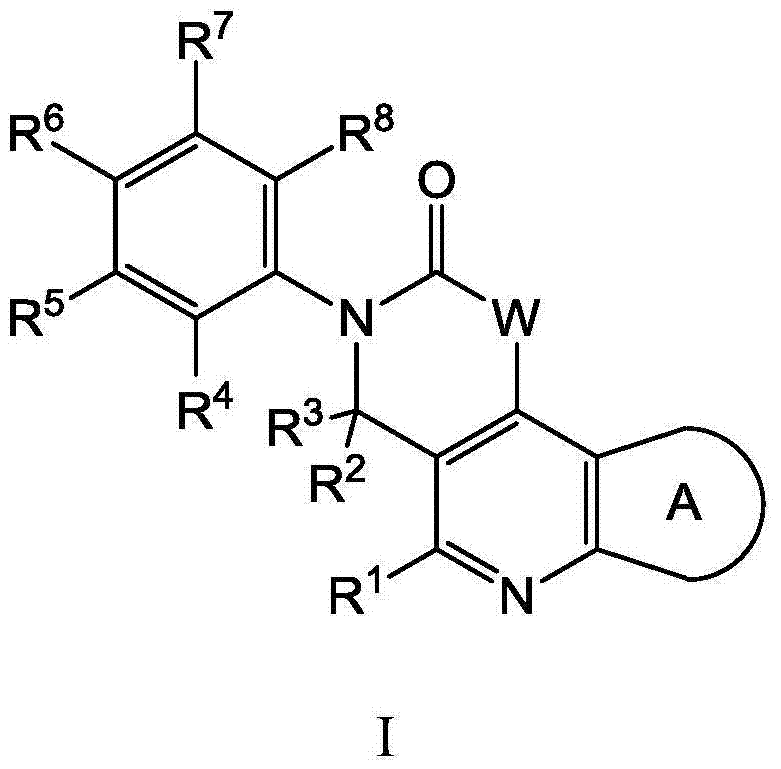
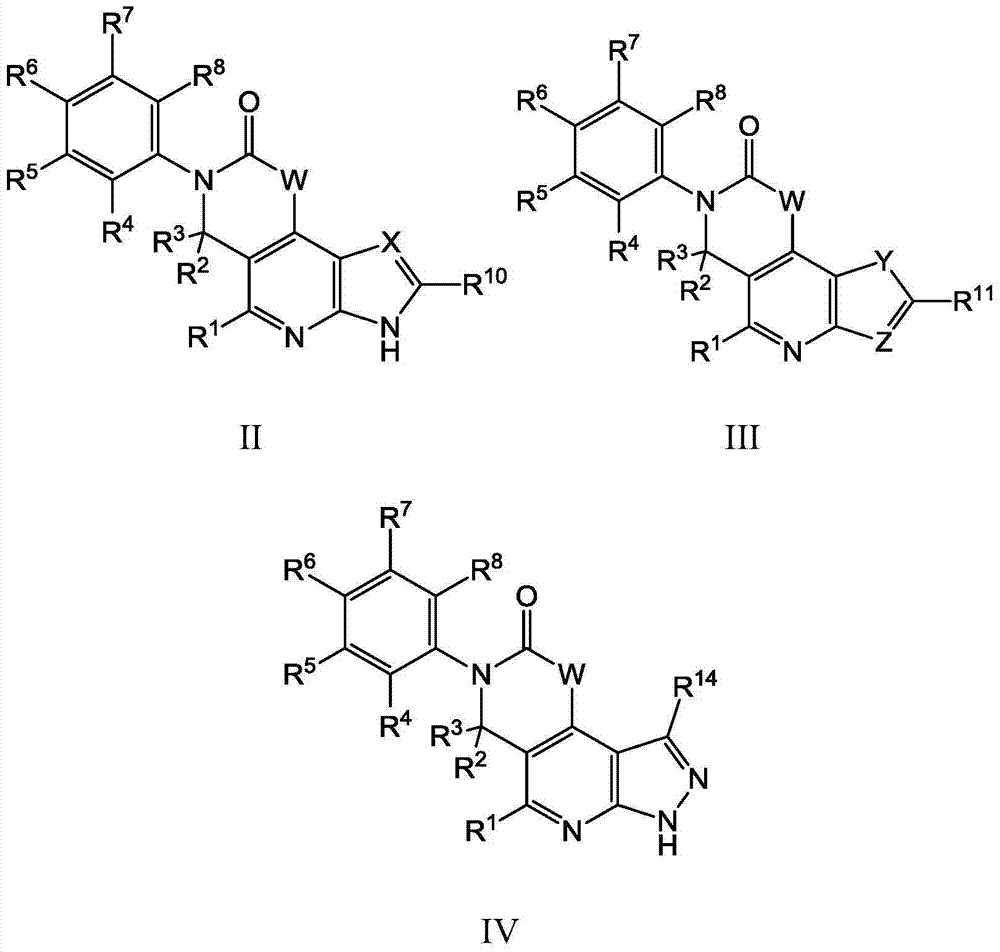
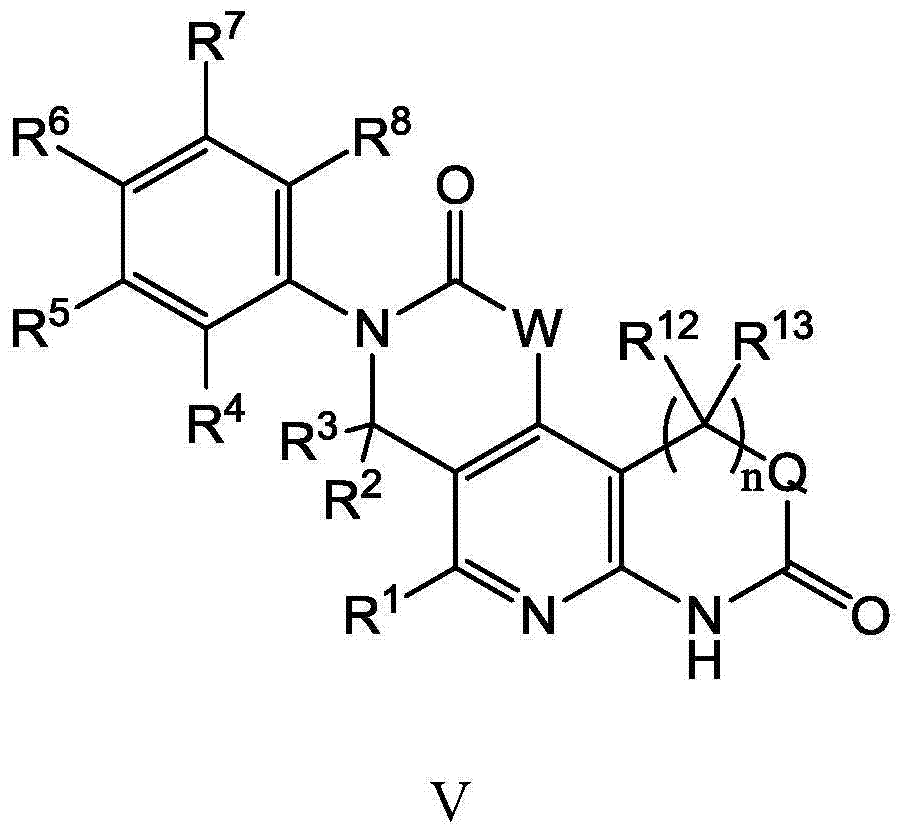
Substituted tricyclic compounds as FGFR inhibitors
A technology of compounds and substituents, applied in the field of tricyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0449] 3-(3,5-Dimethoxyphenyl)-1-methyl-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4 ,3-d]pyrimidin-2-one
[0450]
[0451] Step 1: 4-(Methylamino)-1H-pyrrolo[2,3-b]pyridine-5-carbaldehyde
[0452]
[0453] Heated 4-chloro-1H-pyrrolo[2,3-b]pyridine-5-carbaldehyde (CAS No. 958230-19-8) (from Adesis, Cat. No. 4-263; Synnovator, Cat. No. PBN2011188: 2.71 g, 15 mmol ) and methylamine (33 wt.% in ethanol, 24 mL, 200 mmol) in 2-methoxyethanol (6 mL) to 110 °C and stirred overnight in a sealed pressure flask. The reaction mixture was then cooled to room temperature and concentrated. The residue was dissolved in HCl solution (1 N, 25 mL) and heated to 50 °C. After stirring for 2 hours, the reaction mixture was cooled to room temperature and washed with saturated NaHCO 3 Solution neutralization. The pale yellow precipitate was collected by filtration, washed with water and hexanes, then dried in vacuo to afford the desired product (2.54 g, 97%) as a pale yellow solid. C...
Embodiment 2
[0460] 3-(3,5-Dimethoxyphenyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d] pyrimidin-2-one
[0461]
[0462] This compound was prepared using a procedure similar to that described for Example 1, substituting ammonium hydroxide solution for methylamine and raising the reaction temperature in Step 1 to 130°C. C 17 h 17 N 4 o 3 LC-MS calculated value: [M+H] + m / z: 325.1; found value: 325.1.
Embodiment 3
[0464] 3-(3,5-Dimethoxyphenyl)-1-ethyl-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4 ,3-d]pyrimidin-2-one
[0465]
[0466] This compound was prepared using a procedure similar to that described for Example 1, substituting ethylamine (2M in THF) for methylamine and raising the reaction temperature in Step 1 to 130°C. C 19 h 21 N 4 o 3 LC-MS calculated value: [M+H] + m / z: 353.2; Found: 353.1. 1 H NMR (500MHz,DMSO)δ12.18(s,1H),8.12(s,1H),7.58–7.53(m,1H),6.75(d,J=2.9Hz,1H),6.56(d,J= 2.2Hz, 2H), 6.42(t, J=2.2Hz, 1H), 4.86(s, 2H), 4.21(q, J=6.9Hz, 2H), 3.75(s, 6H), 1.38(t, J= 6.9Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com