A kind of purification method of whey protein
A purification method and technology of whey protein, which is applied in the field of animal endogenous α-lactalbumin and β-lactoglobulin, can solve the problems of protein loss, activity loss, and high cost of chromatography technology, and achieve large-scale production and good quality. separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Collect mammalian milk samples containing recombinant human α-lactalbumin (0.1-5 g / L), including milk samples from transgenic animals containing recombinant human α-lactalbumin, or milk samples mixed with recombinant human α-lactalbumin Non-transgenic animal milk samples with albumin, among which milk samples are preferably fresh liquid milk samples, or liquid milk samples obtained after thawing frozen samples or reconstituted milk powder. Mammalian milk samples include cow milk, goat milk, rabbit milk etc., milk is preferred. Degrease the above milk sample through a butterfly centrifuge, and use 0.8-1.4μm ceramic membrane microfiltration technology to remove bacteria in milk. The operating temperature is 0-35°C, and the inlet pressure is 0.38MPa. The operating pressure is set to 0.15MPa, the flow rate is 5.8m / s, and the flux is 350L m 2 / h (LMH). Then remove the casein in the skim milk through the 80nm-1.4μm ceramic membrane, set the operating pressure at 0.05-0.2MPa...
Embodiment 2
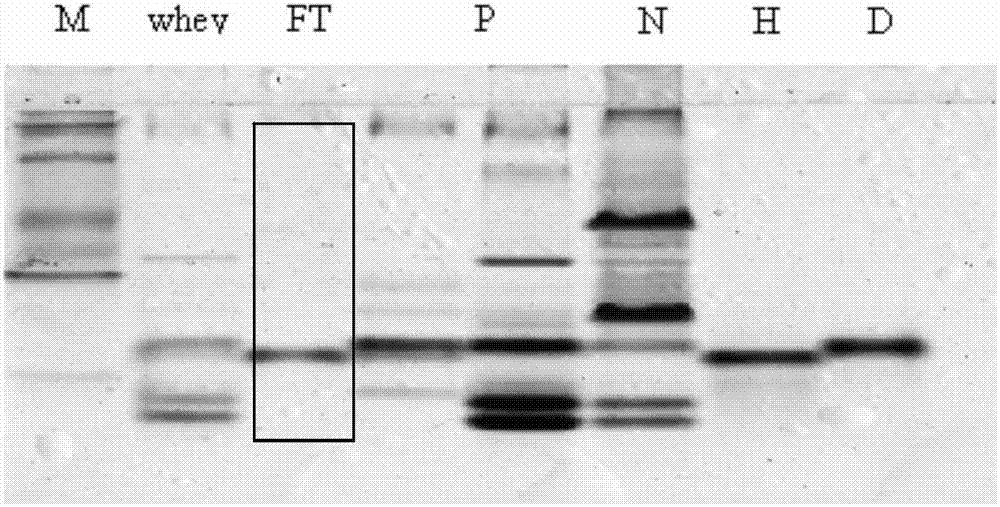
[0036] The whey prepared in Example 1 was loaded onto a DEAESepharose Fast flow anion-exchange chromatography column equilibrated with 60mM, pH6.5 Tris-Cl buffer solution, the flow rate was 10-100ml / min, and the whey containing recombinant human α-lactalbumin was collected. The breakthrough solution, the purity of recombinant human α-lactalbumin obtained is ≥85%, and the yield is ≥90%; use 60mM, pH6.5 Tris-Cl buffer to wash the chromatography column to the baseline, and use a conductivity of 50-84ms / cm of NaCl, 60mM, pH6.5 Tris-Cl buffer solution to elute the components containing bovine α-lactalbumin and β-lactoglobulin and collect them. 12% Native-PAGE electrophoresis results of DEAE medium anion exchange chromatography fraction figure 1 . It can be seen that bovine endogenous α-lactalbumin and recombinant human α-lactalbumin are effectively separated by anion exchange chromatography, and bovine α-lactalbumin and β-lactoglobulin are all eluted.
Embodiment 3
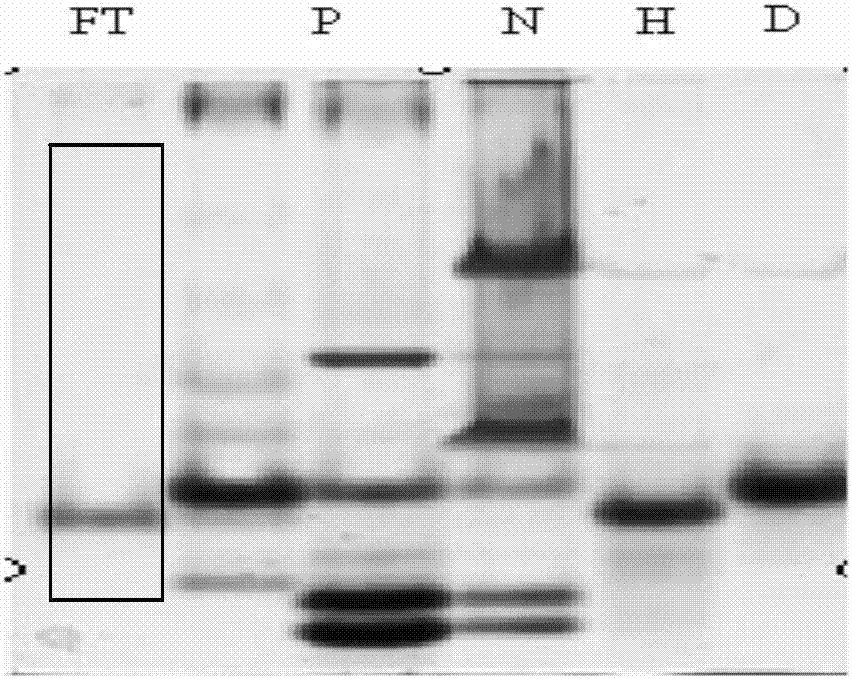
[0038] The whey prepared in Example 1 was loaded onto the Q SepharoseFast flow anion exchange chromatography column equilibrated with 50mM, pH7.0 Tris-Cl buffer solution, the flow rate was 10-100ml / min, and the whey containing recombinant human α-lactalbumin was collected. For the breakthrough solution, the protein purity is ≥80%, and the recovery rate is ≥90%. Use 50mM, pH7.0 Tris-Cl buffer to wash the column to the baseline, and use NaCl with a conductivity of 50-84ms / cm, 50mM, pH7. 0 Tris-Cl buffer to elute the miscellaneous proteins containing bovine α-lactalbumin and β-lactoglobulin and collect them. The 12% Native-PAGE electrophoresis results of Q medium anion exchange chromatography fractions figure 2 . Thus, it can be known that bovine endogenous α-lactalbumin and recombinant human α-lactalbumin can be separated by anion exchange chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com