Reagent for hair water soy sauce detection and preparation method thereof
A hair water and reagent technology, which is applied in biological testing, material inspection products, etc., can solve problems such as difficulty in distinguishing hair water and soy sauce, and achieve the effects of short detection time, obvious effect and simple use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
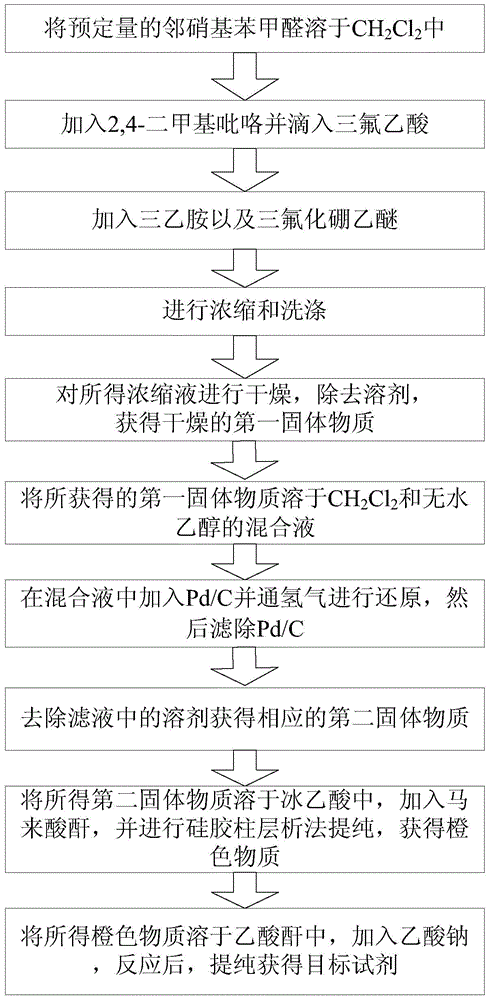
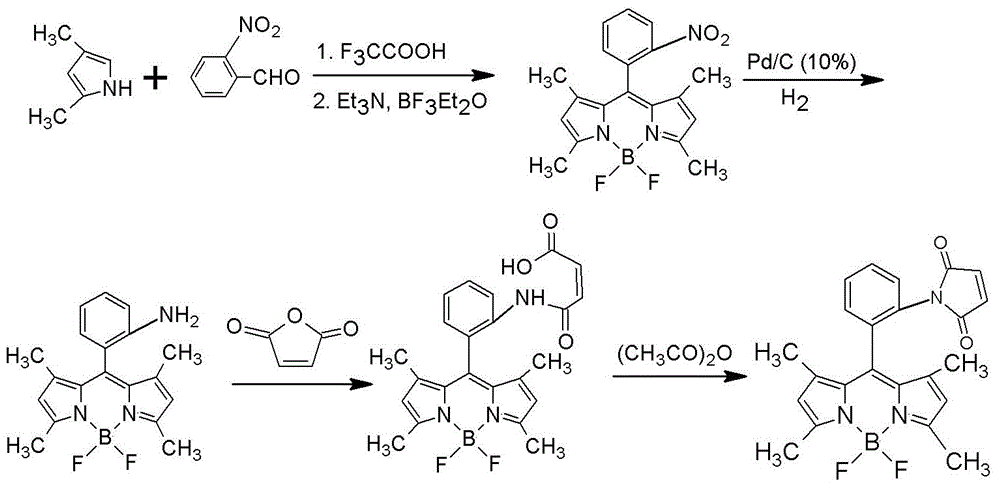
[0037] Combine below figure 1 and 2 As shown, the synthesis process of the reagent for the detection of hair water and soy sauce in this embodiment is introduced in detail.
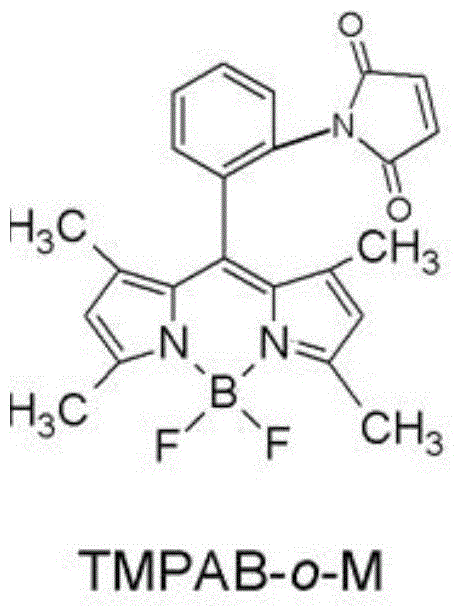
[0038] In this example, by first synthesizing 1,3,5,7-tetramethyl-8-phenyl-(2-nitro)-boron difluoride dipyrromethane (TMPB-o-N), and then synthesizing 1, 3,5,7-Tetramethyl-8-phenyl-(2-amino)-borondifluoride dipyrromethane (TMPB-o-A), and finally the test reagent of the present invention was obtained.
[0039] Specifically, first, accurately weigh 1.06 g (7.0 mmol) of o-nitrobenzaldehyde, dissolve it in 500 mL of dry CH 2 Cl 2 .
[0040] Next, under nitrogen protection, 1.44 mL (14.0 mmol) of 2,4-dimethylpyrrole was slowly added dropwise to the obtained solution while stirring. Afterwards, 1 drop of trifluoroacetic acid was added dropwise, the resulting solution was sealed, and kept stirring overnight at 25° C. until the substance spots of o-nitrobenzaldehyde had disappeared by detection by thin-layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com