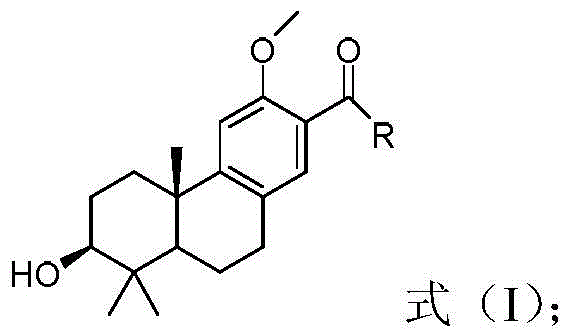
Tricyclic diterpene derivatives, preparation method thereof, and application thereof in the preparation of antitumor drugs
An anti-tumor drug and a technology for tricyclic diterpenes, which are applied in the fields of medicine and their preparation and application, can solve the problems of low content of diterpenoids, restrict in-depth research and clinical application, etc. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of compound shown in formula (5)
[0040]
[0041] The preparation of tricyclic diterpene derivatives shown in formula (5), namely compound 5: Dissolve compound 1 (8g, 29.19mmol) in 50ml dichloromethane, slowly add Br 2 (1.5ml, 29.19mmol) of dichloromethane solution (50ml), after the dropwise addition, stir for 1h, TLC detects that the reaction of raw materials is complete, add water 50ml, extract the aqueous phase with dichloromethane (30ml×3), combine the organic phases, Wash with water (30ml×2), saturated NaHCO 3 Wash (30ml×2), wash with saturated brine (30ml×2), dry over anhydrous sodium sulfate, and concentrate to give compound 2 as a yellow oil, which is directly used in the next step.
[0042] The previous step crude product compound 2 (10.2g, 29mmol), TBSCl (6.5g, 43.5mmol), imidazole (3.95g, 58mmol) were placed in a single-necked bottle, DMF50ml was added, N 2 Replace and stir overnight at room temperature. TLC detects that t...
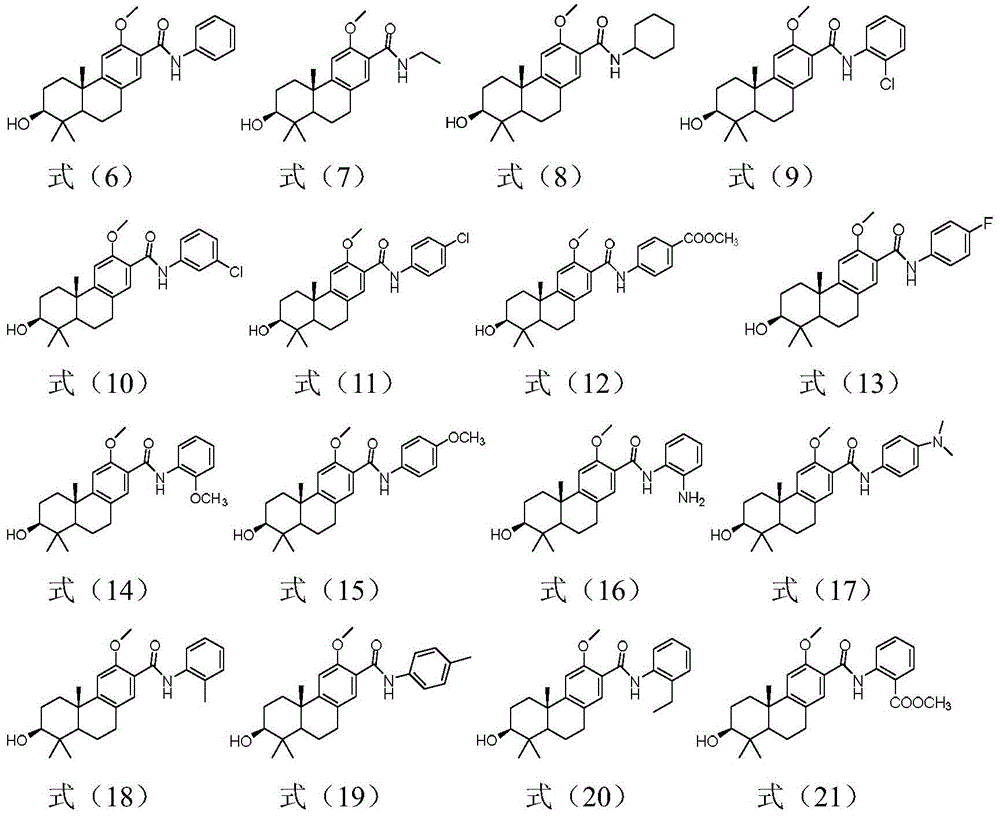
Embodiment 2
[0045] Embodiment 2: formula (6), (7), (8), (9), (10), (11), (12), (13), (14), (15), (16), ( Preparation of Tricyclic Diterpene Derivatives Shown in 17), (18), (19), (20), (21)
[0046]
[0047] The preparation of tricyclic diterpene derivatives shown in formula (6), namely compound 6: Compound 5 (100mg, 0.314mmol), EDC HCl (120.4mg, 0.628mmol), HOBt (84.8mg, 0.628mmol), DMAP ( 153.2mg, 1.256mmol) and aniline (88mg, 0.942mmol) were placed in a single-necked bottle, N 2 For replacement, 10ml of anhydrous dichloromethane was added, and stirred at room temperature overnight. TLC detects that the reaction of the raw materials is complete, add 10ml of water, adjust the pH of the system to 1 H NMR (400MHz, CDCl 3 )δ9.80(s,1H),7.96(s,1H),7.66(d,J=7.9Hz,2H),7.35(t,J=7.8Hz,2H),7.11(t,J=7.4Hz, 1H), 6.87(s, 1H), 4.01(s, 3H), 3.33(dd, J=11.1, 5.0Hz, 1H), 3.07–2.76(m, 2H), 2.30(dt, J=12.9, 3.1Hz ,1H),2.00–1.67(m,4H),1.67–1.58(m,1H),1.37–1.29(m,1H),1.23(s,3H),1.09(s,3H),0.92(s,3H )....
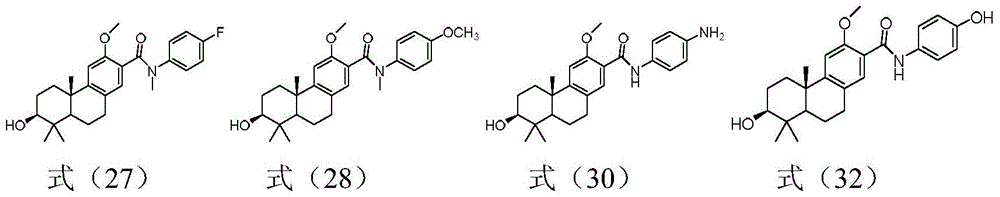
Embodiment 3
[0063] Embodiment 3: the preparation of tricyclic diterpene derivative shown in formula (27), (28)
[0064]
[0065] The preparation of tricyclic diterpene derivatives shown in formula (27), that is, compound 27: compound 13 (110 mg, 0.26 mmol), tert-butyldimethylsilyl chloride (201.4 mg, 1.33 mmol), imidazole (181.7 mg, 2.67 mmol) placed in a single-necked bottle, N 2 For replacement, inject 10ml of anhydrous DMF and stir overnight at room temperature. TLC detected that the reaction of raw materials was complete, added 10ml of water, extracted the aqueous phase with ethyl acetate (10ml×3), combined the organic phases, washed with water (10ml×2), washed with saturated brine (10ml×2), dried over anhydrous sodium sulfate, concentrated , silica gel column chromatography (PE:EA=10:1), concentrated to give compound 23 (99mg, 70.9%)
[0066] Compound 23 (168 mg, 0.319 mmol) obtained from the above reaction was placed in a single-necked bottle, N 2 For replacement, inject 10ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com