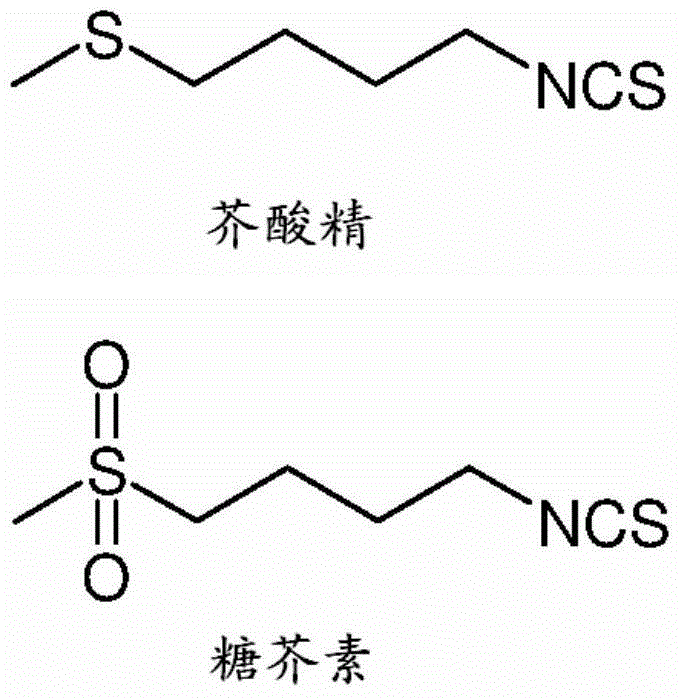
Sulforaphane isolation and purification
A technology of sulforaphane and cyclodextrin, which is applied in organic chemistry, active ingredients of esters, active ingredients of nitrile/isonitrile, etc., can solve the problems of difficult manufacture and distribution of sulforaphane, and achieve the effect of simple cost-effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] This example relates to an embodiment of the invention demonstrating an improved method for purifying sulforaphane.
[0096] A sample of sulforaphane (production department: Beijing Hunda Qunxing Science and Technology Co.; batch #20091101) (8.23 g, 11.3 mmol of sulforaphane, 24.3% purity) was loaded into a 250 mL round bottom flask equipped with a magnetic stirrer And add 43.9 mL of 0.257M cyclodextrin solution (manufacturer: Wacker; batch #60F212; 11.3 mmol of cyclodextrin). The cyclodextrin solution was prepared by heating 288.9 g of cyclodextrin in 1 L of water at 70°C using ultrasound to give a total volume of 1155 mL after the cyclodextrin was completely dissolved.
[0097] The mixture was stirred at room temperature for one hour, sonicated for 10 minutes, and then stirred for another two hours. A small amount of precipitate formed. Stirring was continued overnight, which caused an increase in the amount of precipitate. The mixture was cooled in a refrigerator at 4°...
Embodiment 2
[0102] This example relates to a further embodiment of the invention demonstrating an improved method for purifying sulforaphane.
[0103] A sample of sulforaphane (production department: Beijing Hunda Qunxing Science and Technology Co.; batch #20091101) (3.2 g, 11.3 mmol of sulforaphane, 62.9% purity) was loaded into a 250 mL round bottom flask equipped with a magnetic stirrer And add 43.9 mL of 0.257M cyclodextrin in water to the flask. The cyclodextrin solution was prepared by heating 288.9 g of cyclodextrin in 1 L of water at 70°C using ultrasound to give a total volume of 1155 mL after the cyclodextrin was completely dissolved.
[0104] The solution was stirred at room temperature for 1 hour, sonicated for 10 minutes, and then stirred for another 2 hours. After the precipitate formed, the mixture was cooled overnight at 4°C in the refrigerator. After more solids precipitated, the mixture was filtered to give 11.9 g (92% yield, 96% HPLC purity) of light yellow solid. HPLC an...
Embodiment 3
[0109] The sulforaphane-cyclodextrin complex (2.2 g, 1.9 mmol) obtained in Example 2 was loaded into a 20 mL scintillation vial and 6 mL of water was added. The mixture is sonicated at about 70°C until the dissolution of the solid particles is complete. The tube was then removed from the ultrasonic bath and kept at room temperature for one hour. Solids began to precipitate out of solution. The tube was moved to a refrigerator at 4°C for 30 minutes and filtered to give an off-white solid, which was dried in a vacuum oven at 30°C overnight. Recrystallization yielded 1.5 g of off-white solid (68% yield, 98% HPLC purity).
[0110] 1 HNMR(D 2 O,400MHz); δ2.01(br,4H),2.78(s,3H),3.01(br,2H), 3.65(m,12H), 3.75(br,2H), 3.91(m,24H), 5.15 (d, 6H).
[0111] 13 CNMR(D 2 O, 100MHz); δ129.98, 101.79, 81.39, 74.01, 71.95, 71.83, 60.31, 52.01, 44.95, 37.04, 29.33, 20.08.
[0112] 13 C NMR analysis and 1 Both H NMR analysis (Figure 8 and Figure 9) are consistent with the 1:1.02 ratio of sulforaph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com