Compounded analgesic preparation containing cobratide and oxycodone
A technology of oxycodone and cobotide, which is applied in the field of medicine, can solve the problems of poor stability, easy to cause allergic reactions, and slow onset of action, and achieve the effects of reduced toxic and side effects, strong analgesic effect, and fast peak time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
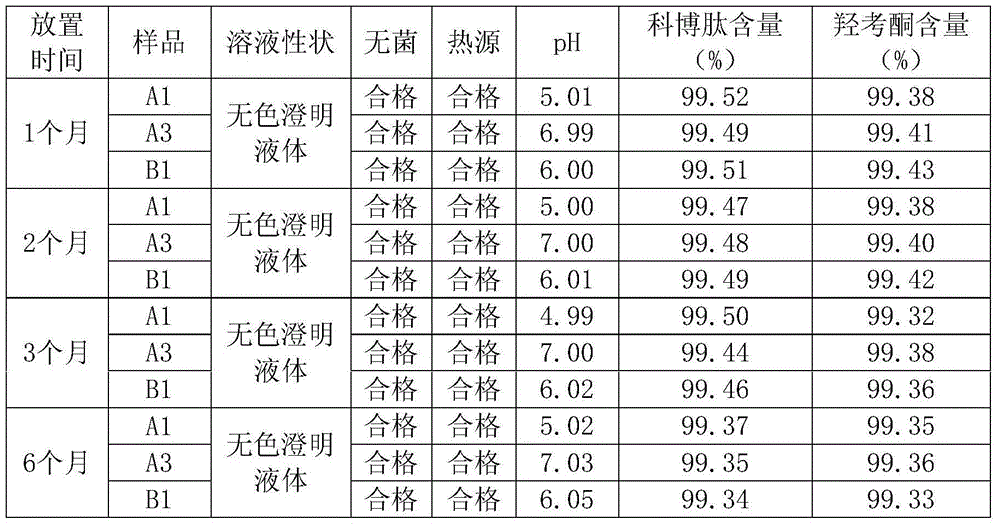
[0022] Embodiment 1: small volume injection: A1
[0023] Take 800ml of water for injection, add potassium hydroxide to adjust the pH value to 5, add 9g of sodium chloride and stir, heat to 35°C, add 0.016g of cobotide, 1.6g of oxycodone, and then add 0.2g of Tween-80 To aid dissolution, stir until the solution is clear, add water for injection with adjusted pH value to 1000ml, filter and sterilize, and pack into ampoules to obtain 400 small-volume injections. The specification is 2.5ml each, containing 40μg of effective dose of Cobotide and 4mg of Oxycodone.
Embodiment 2
[0024] Embodiment 2: small volume injection: A2
[0025] Take 800ml of water for injection, add sodium hydroxide to adjust the pH value to 6, add 8g of sodium chloride and stir, heat to 37°C, add 0.024g of cobotide, 3.2g of oxycodone hydrochloride, and then add 0.16g of Tween- 80 to aid dissolution, stir until the solution is clear, then make up water for injection with adjusted pH value to 1000ml, filter and sterilize, and pack into ampoules to obtain 400 small-volume injections. The specification is 2.5ml each, containing 60μg of effective dose of cobotide and 8mg of oxycodone.
Embodiment 3
[0026] Embodiment 3: small volume injection: A3
[0027] Take 700ml water for injection, add sodium carbonate to adjust the pH value to 7, add 10g sodium chloride and stir, heat to 36°C, add 0.032g cobotide, 4.8g oxycodone hydrochloride active ingredient, and then add 0.24g porol Sham 188, stirred until the solution is clear, then supplemented with water for injection with adjusted pH value to 1000ml, filtered and sterilized, and divided into ampoules to obtain 400 small-volume injections. The specification is 2.5ml each, containing 80μg of effective dose of Cobotide and 12mg of Oxycodone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com