Application of an improved fischer-type glycosylation reaction in the construction of glycosidic bonds in saponins
A technology of glycosidic bonds and saponins, which is applied in the field of construction strategies of glycosidic bonds in saponins, can solve the problems of increasing production costs and unfavorable industrial production, and achieve the effect of easy operation, easy industrial production, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
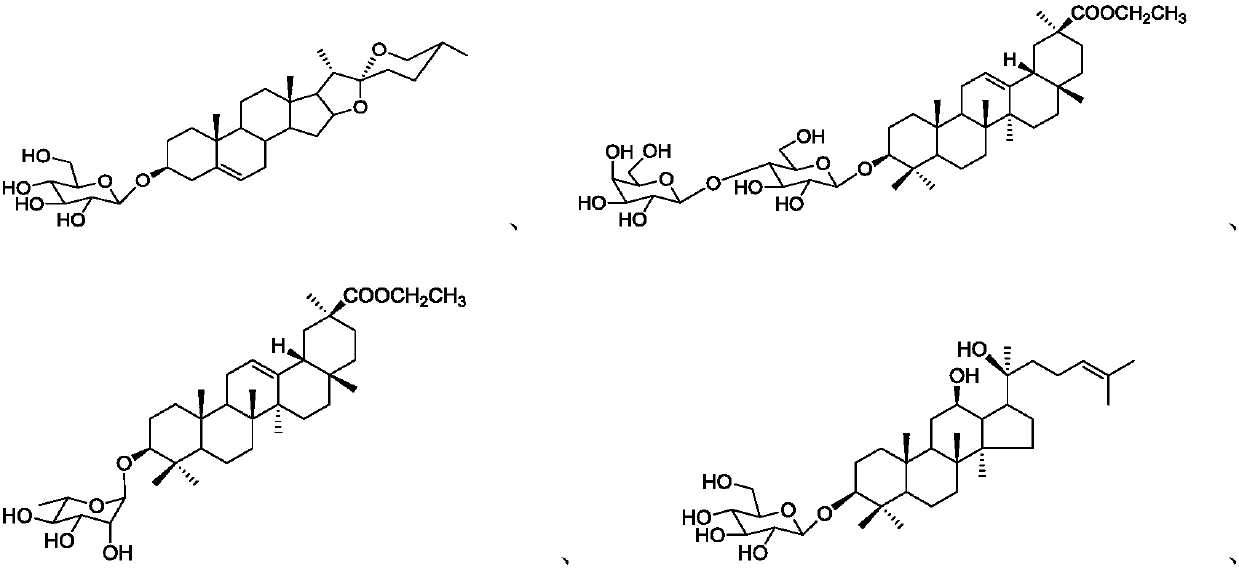
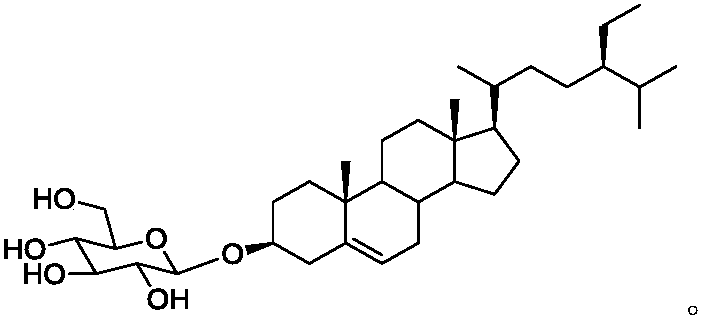
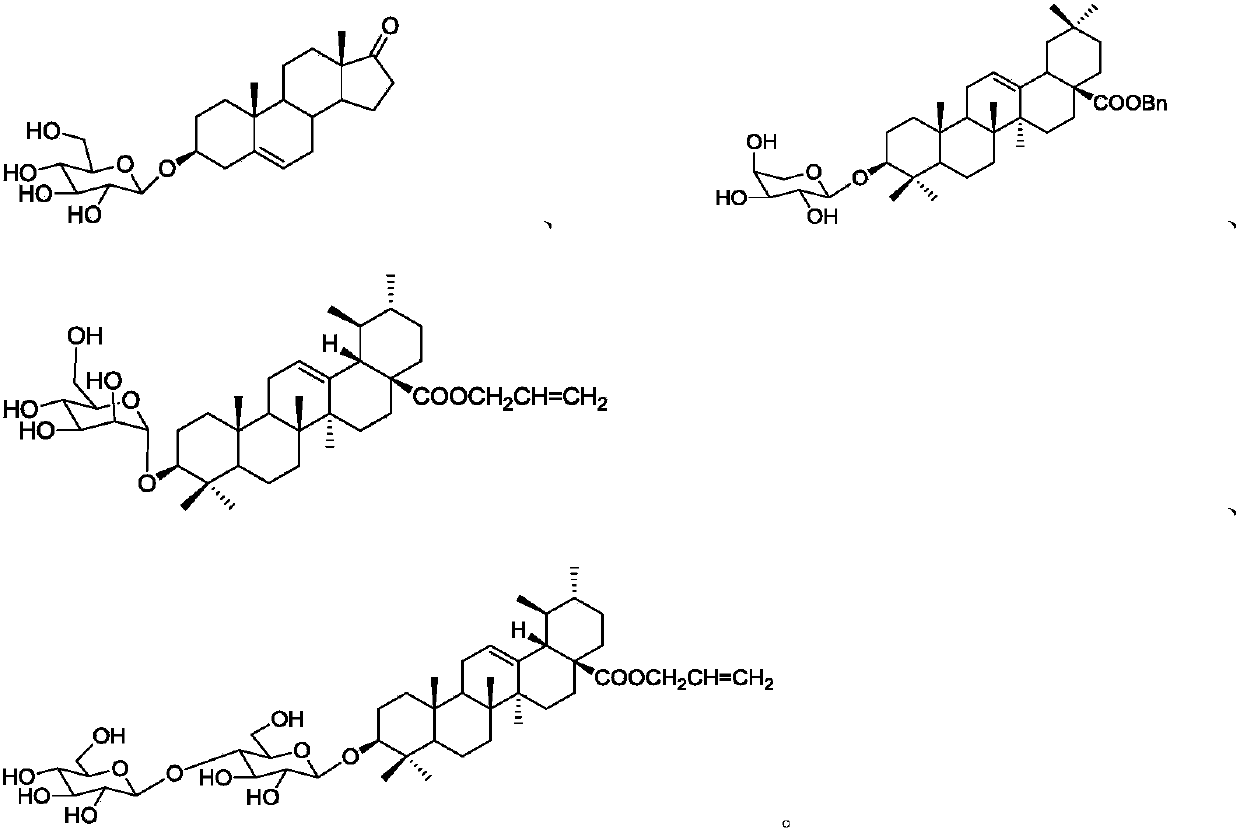
Image
Examples
Embodiment 1
[0028] 3-O-β-D-Glucopyranose-DHEA ( )Synthesis
[0029] Weigh dehydroepiandrosterone (34.6g, 0.12mol), D-glucose (18.0g, 0.1mol) was dissolved in anhydrous toluene (200mL), at 80 ° C, add H 2 SO 4 -SiO 2 (250mg, 0.5mmol), react at a constant temperature under the protection of argon until the D-glucose almost completely disappears (about 5h) as detected by TLC. Methane is used as the eluent, and TLC detects until there is no dehydroepiandrosterone in the eluent, and the eluate is concentrated to obtain dehydroepiandrosterone (14.4g, 0.05mol), and ethyl acetate is used as the eluent to continue Elution, TLC detection until there is no title compound in the eluate, the eluate is concentrated to obtain 22.5 g of white solid, which is the title compound, the yield is 50.0%, and the purity by HPLC is about 98.6%.
[0030] Structure confirmation data: ESI-MS (m / z): 473.3[M+Na l + , 1 H NMR (400MHz, CD 3 OD): δ5.42(d, J=5.2Hz, 1H, H-6), 4.83(d, J=1.4Hz, 1H, H-1), 3.75(dd, J=3...
Embodiment 2
[0035] Trillin (trillin, CAS registration number: 14144-06-0, structural formula: )Synthesis
[0036] Weigh diosgenin (62.2g, 0.15mol), D-glucose (18.0g, 0.1mol) was dissolved in THF (300mL), add TfOH-SiO 2 (500mg, 1.0mmol), reflux reaction, until TLC detection D-glucose almost completely disappeared (about 12h), after the reactant was concentrated, through silica gel column chromatography (silica gel 200~300 mesh), first use dichloromethane as washing Remove agent, TLC detects until there is no diosgenin in the eluent, concentrates the eluent to obtain diosgenin (16.6g, 0.04mol), then continues eluting with ethyl acetate as eluent, TLC detects until eluent There was no trillium in the eluate, and the eluate was concentrated to obtain trillium (46.1 g), with a yield of 80% and a purity of 96.5% by HPLC.
[0037] Structure confirmation data: Melting point: 275-280°C, ESI-MS (m / z): 577.5[M+H l + , 1 HNMR (400MHz, CD 3 OD) δ: 0.64 (3H, d, J = 5.1Hz, CH 3 -27),0.78(3H,s,CH ...
Embodiment 3
[0039] 3-O-β-L-arabinopyranose-oleanolic acid benzyl ester ( )Synthesis
[0040] Weigh benzyl oleanolic acid (136.7g, 0.25mol), L-arabinose (15.0g, 0.1mol) was dissolved in dioxane (500mL), at 100 ° C, add HClO 4 -SiO 2 (1.0g, 2.0mmol), constant temperature reaction, until the TLC detection of L-arabinose almost completely disappeared (about 2h), after the reaction was concentrated, silica gel column chromatography (silica gel 200 ~ 300 mesh), first with dichloromethane As eluent, TLC detects until there is no benzyl oleanolic acid in the eluent, and the eluent is concentrated to obtain benzyl oleanolic acid (65.6g, 0.12mol), and ethyl acetate is used as the eluent The eluent was continuously eluted and detected by TLC until there was no title compound in the eluate, and the eluate was concentrated to obtain the title compound (48.9 g), with a yield of 72% and a purity of 97.3% by HPLC.
[0041] Structure confirmation data: ESI-MS (m / z): 701.5[M+Na l + , 1 H NMR (400MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com