Markers and detection reagents for postpartum eclampsia diagnosis
A postpartum eclampsia and kit technology, applied in the field of markers and detection reagents, can solve the problems of differences in clinical manifestations of patients, limited understanding of disease pathogenesis and basic knowledge of occurrence and development, and achieve the effect of high specificity and strong sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Discovery and verification of clinical diagnostic markers for postpartum eclampsia
[0030] 1.1 Selection and grouping of research objects
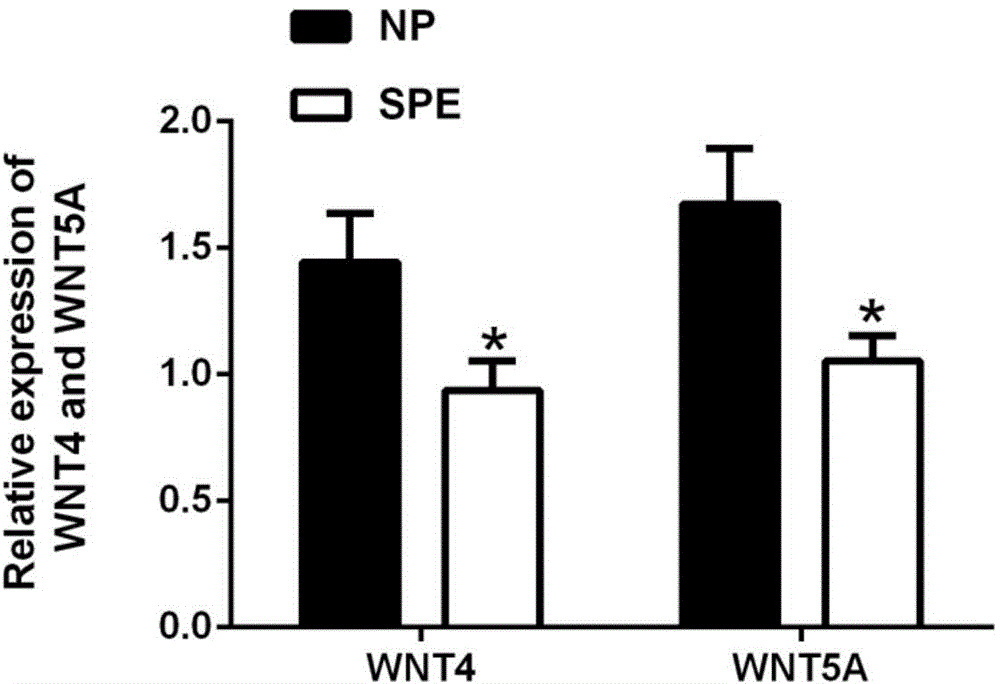
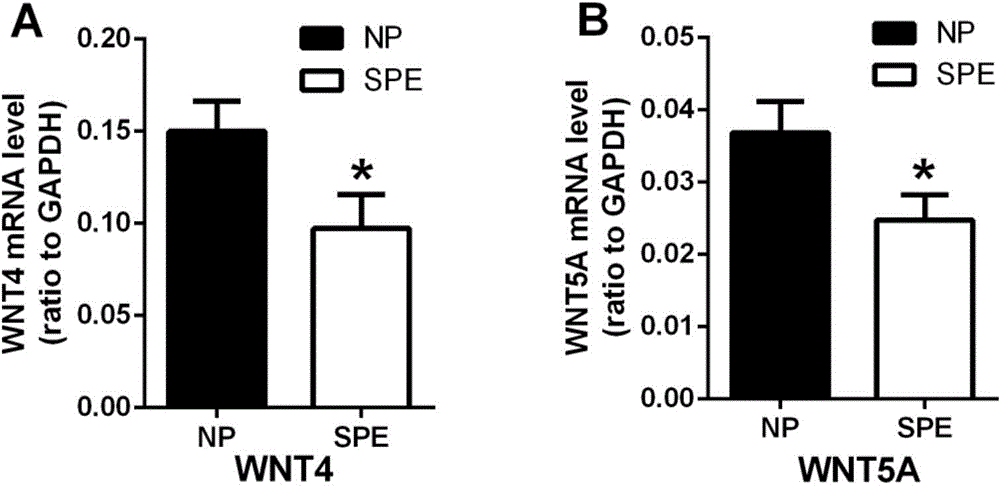
[0031] The research objects of this example were all 40 women who underwent cesarean section in the Obstetrics and Gynecology Department of Qilu Hospital of Shandong University in Jinan City between January 2014 and January 2015. Including 20 patients with postpartum eclampsia (SPE group), as the postpartum eclampsia group, the average age of this group is 29.40±1.111, and the average gestational age is 36.78±0.625. 20 normal healthy pregnant women were taken as the normal pregnancy group (NP group). The average age of this group was 28.73±0.639, and the average gestational week was 39.10±0.233.
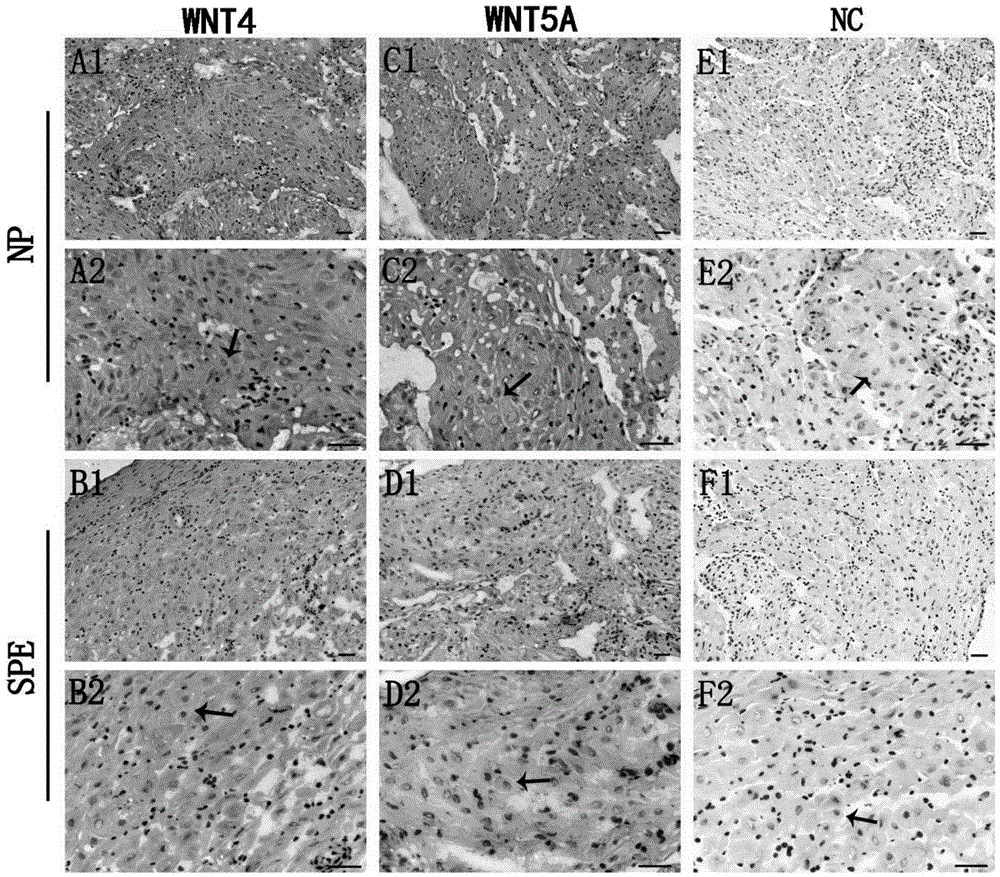
[0032] 1.2 Collection and processing of decidua tissue samples
[0033] 1.2.1 Tissue collection: After the placenta is delivered by cesarean section, use sterile gauze to wipe the uterine wall where the placenta is attached, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com