Hydrophobic drug nanometer capsule of pH response of near-infrared fluorescent trace and preparation method of hydrophobic drug nanometer capsule
A technology of hydrophobic drugs and nanocapsules, which is applied in the preparation of in vivo experiments, drug combinations, microcapsules, etc., can solve the problems of difficult penetration of animal tissues and long time for data collection, and achieve novel and simple preparation methods. The effect of strong fluorescent signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A. the preparation method of oleylamine grafted polysuccinimide:
[0022] Add 1.6g polysuccinimide (PSI) into 32mL N,N-dimethylformamide and heat to 80°C for 1 hour until dissolved; then add 2.1mL oleylamine and maintain 100°C for 4 hours; finally add methanol to dissolve It is precipitated, centrifuged, and then dispersed in 8 mL of chloroform to obtain a chloroform solution of oleylamine grafted polysuccinimide;
[0023] b. Use oleylamine grafted polysuccinimide to encapsulate hydrophobic anticancer drugs and near-infrared fluorescent tracer materials into nanocapsules:
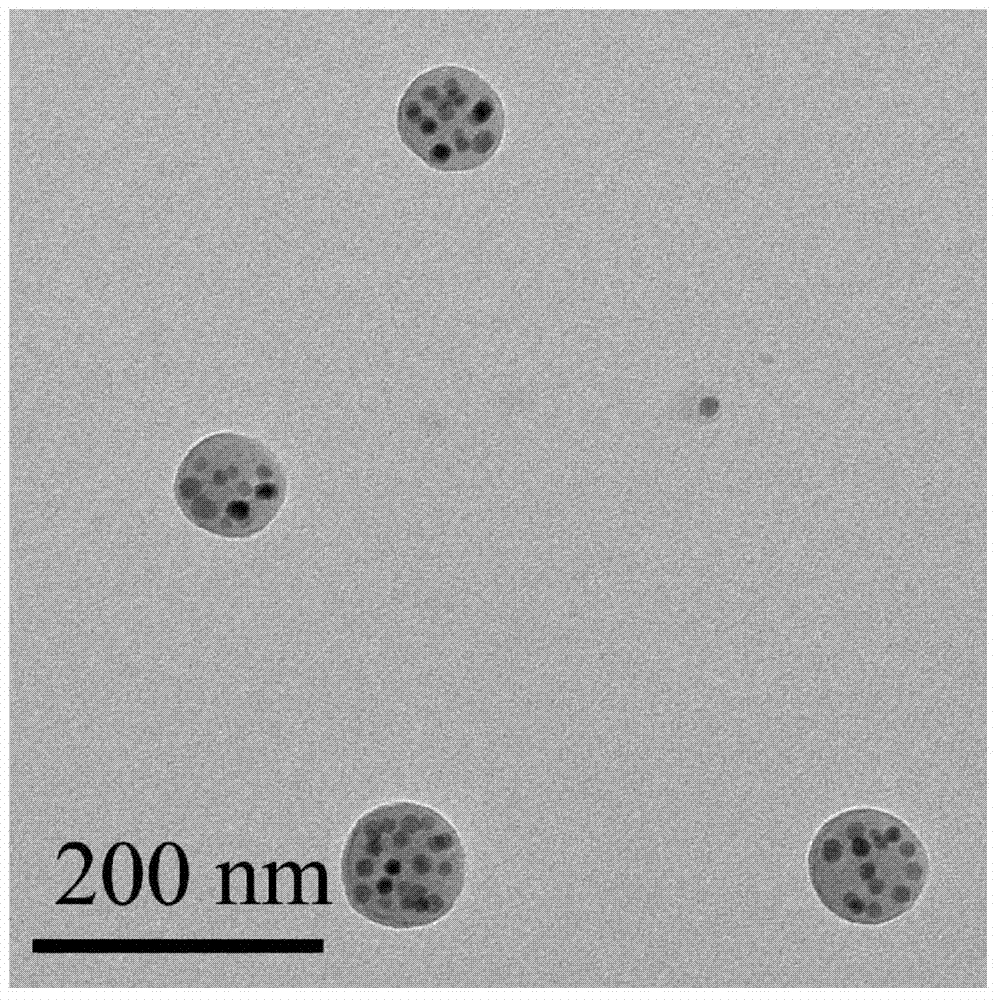
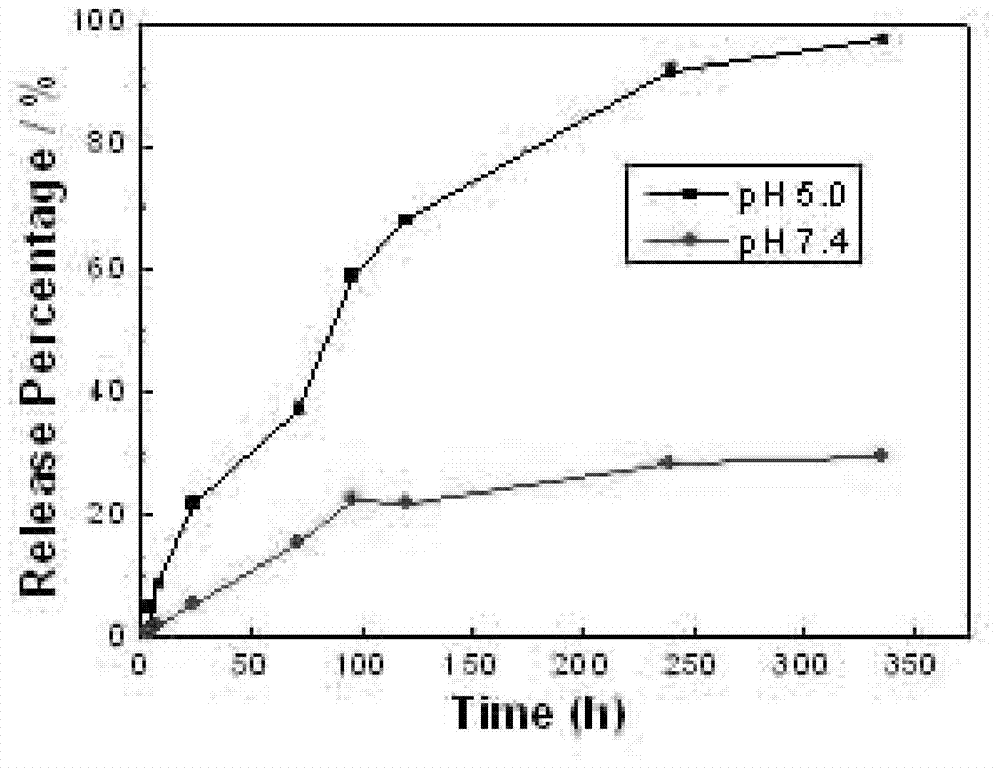
[0024] 5mg poly(ethylene-ethylene glycol), 50μL 0.05mmol / L surface chelated Ag of oleic acid 2 S nanoparticles in chloroform solution, 200 μL oleylamine grafted polysuccinimide in chloroform solution, 1.0 mg paclitaxel, 600 μL chloroform, 10 mL deionized water and 50 μL 0.1 mmol / L NaOH aqueous solution were mixed; 300W power Under ultrasonication for 6 minutes, the obtained emulsion was stirred at ...
Embodiment 2
[0029] A. the preparation method of oleylamine grafted polysuccinimide:
[0030] Add 1.6g polysuccinimide (PSI) into 32mL N,N-dimethylformamide and heat to 80°C for 1 hour until dissolved; then add 2.1mL oleylamine and maintain 100°C for 4 hours; finally add methanol to dissolve It is precipitated, centrifuged, and then dispersed in 8 mL of chloroform to obtain a chloroform solution of oleylamine grafted polysuccinimide;
[0031] b. Encapsulate hydrophobic anticancer drugs into nanocapsules by grafting polysuccinimide with oleylamine:
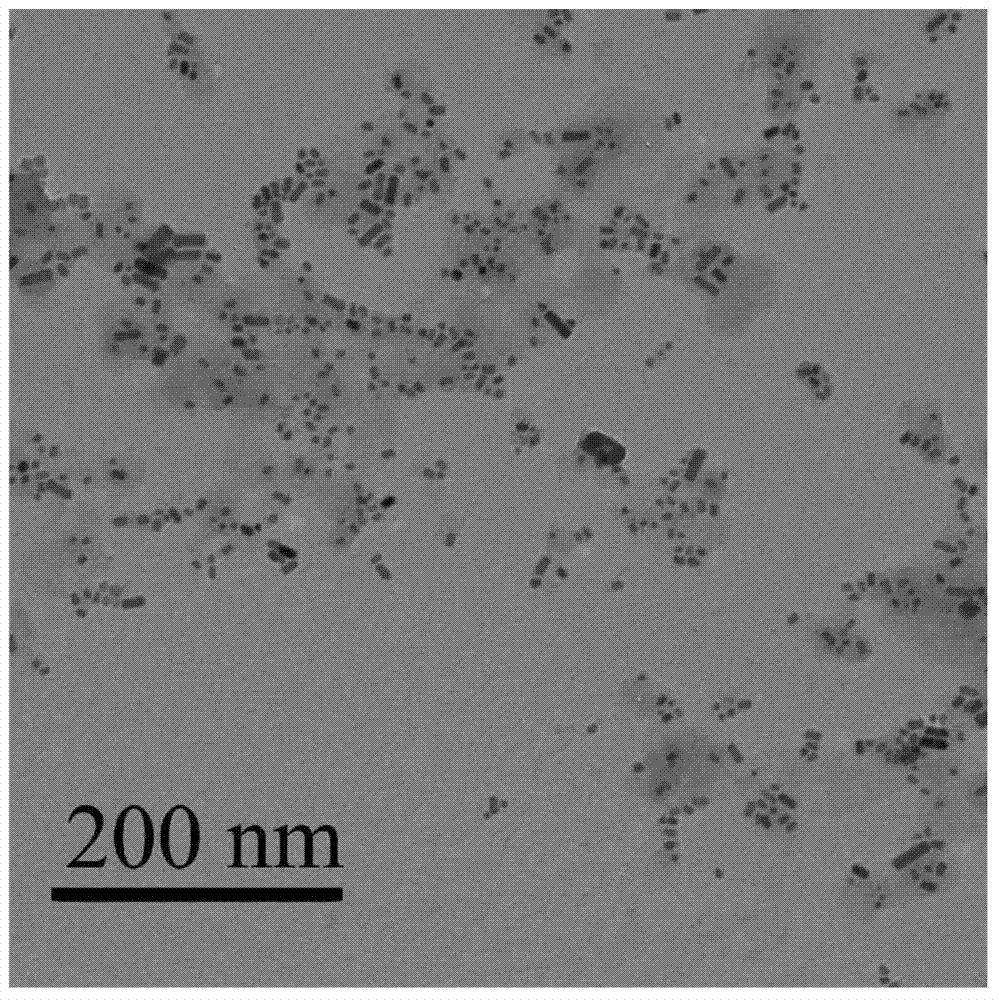
[0032] 5mg poly(ethylene-ethylene glycol), 50 μL 0.05mmol / L surface chelated Ag of dodecanethiol 2 S nanoparticles in chloroform, 200 μL 200 mg / mL oleylamine-grafted polysuccinimide in chloroform, 1.0 mg camptothecin, 600 μL chloroform, 10 mL deionized water and 50 μL 0.1 mmol / L NaOH Mix the aqueous solution; ultrasonicate for 6 minutes at 300W power, and stir the obtained emulsion at 55°C for 2 hours to evaporate chloroform; after the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Light wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap