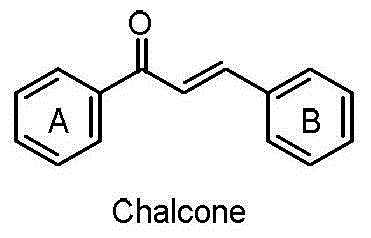
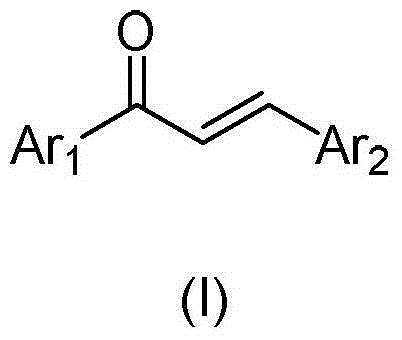
Chalcone analogue containing 2-methyl-4-oxo-quinazoline-6-base, and preparation method and application thereof
A technology of methylquinazoline and oxoquinazoline, which is applied in the application field of chalcone analogues and antitumor drugs, and can solve problems that do not involve 2-methyl-4-oxoquinazoline Chalcone analogues and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] (E)-6-(3-Phenylacryloyl)-2-methylquinazolin-4(3H)-one (Compound 1)
[0125] Yield: 86%, white solid, m.p.280-282℃. 1 H NMR (600MHz, DMSO-d 6 )δ:2.53(s,3H,CH 3 ), 7.48(m, 3H, phenyl 3'-H, 4'-H, 5'-H), 7.81(d, J=15.6Hz, 1H, COCH=), 7.83(d, J=8.4Hz, 1H, quinazolin-4(3H)-one 8-H), 7.95(m, 2H, phenyl 2'-H, 6'-H), 8.06(d, J=15.6Hz, 1H,=CHPh) ,8.55(d,J=8.4Hz,1H,quinazolin-4(3H)-one 7-H),8.84(s,1H,quinazolin-4(3H)-one 5-H),12.96( br s,1H,NH).ESI-HRMS m / z:C 18 h 15 N 2 o 2 ([M+H] + ) calculated value: 291.1134; measured value: 291.1132.
Embodiment 2
[0127] (E)-6-(3-(2-methoxyphenyl)acryloyl)-2-methylquinazolin-4(3H)-one (compound 2)
[0128] Yield: 56%, yellow solid, m.p.256-258℃. 1 H NMR (600MHz, DMSO-d 6 )δ:2.40(s,3H,CH 3 ),3.92(s,3H,OCH 3 ), 7.05(t, J=7.8Hz, 1H, phenyl 5'-H), 7.14(d, J=7.8Hz, 1H, phenyl 3'-H), 7.47(td, J=7.8, 1.2Hz ,1H,phenyl 4'-H),7.70(d,J=8.4Hz,1H,quinazolin-4(3H)-one 8-H),7.98(d,J=15.6Hz,1H,COCH= ), 8.04 (dd, J = 7.8, 1.2Hz, 1H, phenyl 6'-H), 8.10 (d, J = 15.6Hz, 1H, = CHPh), 8.44 (dd, J = 8.4, 2.4Hz, 1H , quinazoline-4(3H)-one 7-H), 8.79(d, J=2.4Hz, 1H, quinazoline-4(3H)-one 5-H), 12.47(s, 1H, NH) .ESI-HRMS m / z:C 19 h 17 N 2 o 3 ([M+H] + ) calculated value: 321.1239; measured value: 321.1238.
Embodiment 3
[0130] (E)-6-(3-(3-methoxyphenyl)acryloyl)-2-methylquinazolin-4(3H)-one (Compound 3)
[0131] Yield: 41%, white solid, m.p.229-231℃.1 H NMR (600MHz, DMSO-d 6 )δ:2.40(s,3H,CH 3 ),3.85(s,3H,OCH 3 ), 7.05(dd, J=7.8, 2.4Hz, 1H, phenyl 4'-H), 7.39(t, J=7.8Hz, 1H, phenyl 5'-H), 7.49(d, J=7.8Hz ,1H,phenyl 6'-H),7.53(s,1H,phenyl 2'-H),7.70(d,J=8.4Hz,1H,quinazolin-4(3H)-one 8-H) , 7.77(d, J=15.6Hz, 1H, COCH=), 8.05(d, J=15.6Hz, 1H,=CHPh), 8.48(dd, J=8.4, 1.8Hz, 1H, quinazoline-4( 3H)-one 7-H), 8.82(d, J=1.8Hz, 1H, quinazolin-4(3H)-one 5-H), 12.48(s, 1H, NH).ESI-HRMS m / z :C 19 h 17 N 2 o 3 ([M+H] + ) calculated value: 321.1239; measured value: 321.1239.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com