Ginsenoside-containing medicine composition
A technology of ginsenoside and composition, applied in the field of medicine, can solve the problem of less reports on the pharmacological activity of Panax notoginseng saponins, and achieve the effect of enhancing the immune response function of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 injection
[0016] Weigh 50g of arginine and water of equal quality, stir evenly, add 100g of ginsenoside Rg3, 62g of ginsenoside Rh2, and 88g of notoginsenoside ft1, heat to 50°C, stir to dissolve, and adjust the pH value with an aqueous solution containing 9% hydrochloric acid to 4.6, add water to the full amount, and stir evenly; then add activated carbon with a liquid volume of 0.1% (g / ml), stir for 25 minutes, and decarbonize by coarse filtration; pass through a 0.22 μm filter membrane to obtain a refined filtrate; potting, each The bottled volume is 2mL; autoclave at 118°C for 18 minutes, inspect, pack, and store.
Embodiment 2
[0017] The preparation of embodiment 2 tablet
[0018] 1) Mix 16 g of ginsenoside Rg3, 10 g of ginsenoside Rh2 and 14 g of notoginsenoside ft1 and stir evenly to obtain a powdery solid;
[0019] 2) Mix the powder obtained in step (1) with 60 g of lactose, 3 g of sodium carboxymethyl starch and 4.5 g of micropowdered silica gel, add 60 g of arginine to make a suitable soft material, and granulate with a 20-mesh sieve;
[0020] 3) Mix 5.5 g of sodium carboxymethyl starch and 2.1 g of micropowder silica gel with the dry granules obtained in step (2), and compress into tablets to obtain tablets.
Embodiment 3
[0021] Embodiment 3 capsule preparation
[0022] Weigh 16g of ginsenoside Rg3, 10g of ginsenoside Rh2, and 14g of notoginsenoside ft1, add 274g of starch, mix well, sieve, use ethanol aqueous solution as a binder to make a suitable soft material, granulate with 18 mesh sieve, 60°C Dry in an oven for 5 hours, sieve through a 16-mesh sieve, add 2 g of magnesium stearate, and mix well to obtain capsules.
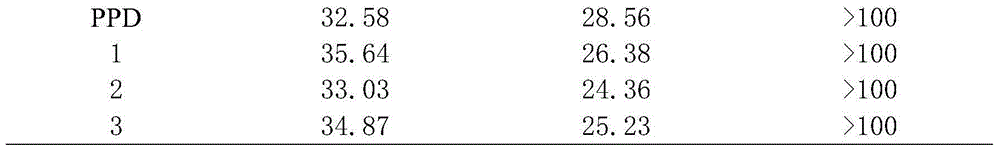
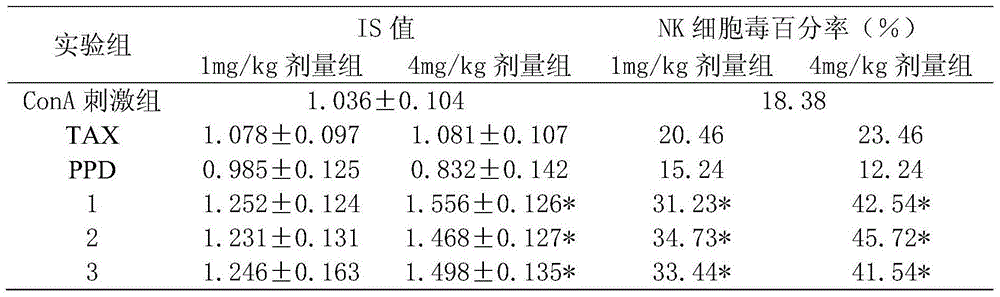
[0023] The application of the composition of the present invention in antitumor will be described below in conjunction with specific experiments.
[0024] Experimental animals: Kunming mice were purchased from the Experimental Animal Center of Kunming Medical College.
[0025] Cells: B16 melanoma cells (B16melanoma cell) and human hepatoma cell (HepG2) cells were purchased from American Type Cell Bank (ATCC, Hanassas, VA, USA).
[0026] Medium: DMEM (FBS; HyClone, Logan, UT, USA) for cell culture, supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 0.03% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com