Preparation method of human T cell carrying anti-Her2/CD3 bispecific function protein
A bispecific antibody and host cell technology, applied to cells modified by introducing foreign genetic material, hybrid immunoglobulins, antibodies, etc., can solve the problems of deletion, residual tumor cell tumor recurrence, etc., and achieve good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] ① Preparation of target gene:
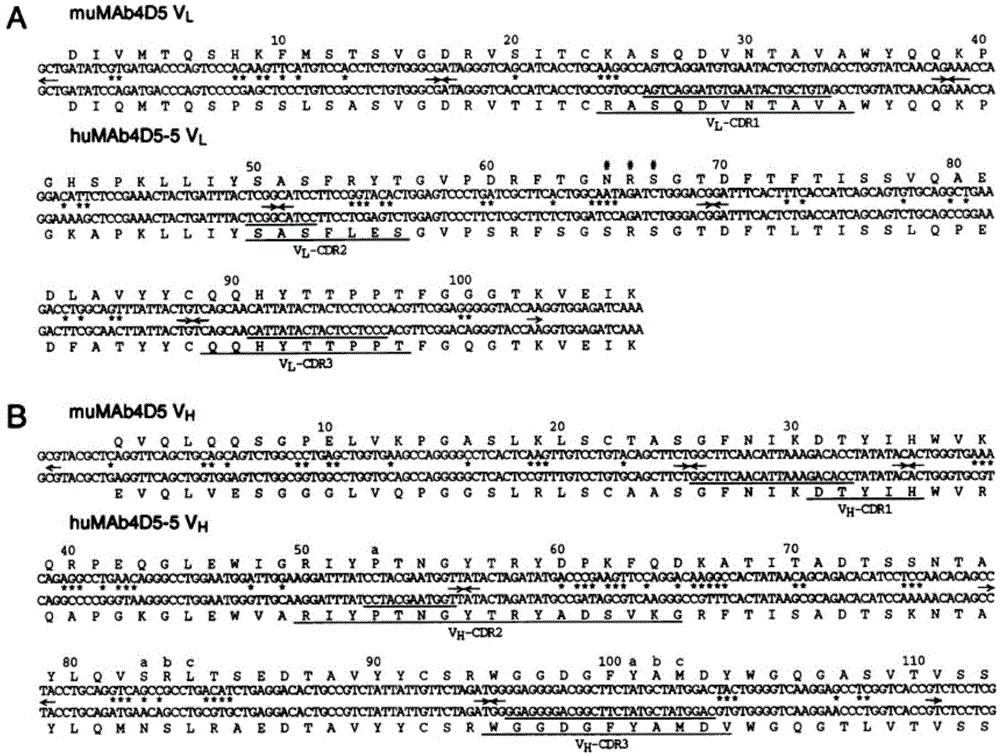
[0062] a. The anti-Her2 antibody sequence is derived from the humanized antibody in the literature (Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA, 1992, 89: 4285-4289.): light chain segment huMAb4D5 -5VL and heavy chain segment huMAb4D5-5VH (such as figure 1 Shown).
[0063] The VL sequence of the anti-Her2 antibody used in the examples of the present invention is as follows:
[0064] The nucleic acid sequence is shown in SEQ ID No. 1:
[0065] ATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAATCAGTGCCTCAGTCATAATGTCCAGAGGAGATATCCAGATGACCCAGTCCCCGAGCTCCCTGTCCGCCTCTGTGGGCGATAGGGTCACCATCACCTGCCGTGCCAGTCAGGATGTGAATACTGCTGTAGCCTGGTATCAACAGAAACCAGGAAAAGCTCCGAAACTACTGATTTACTCGGCATCCTTCCTTGAGTCTGGAGTCCCTTCTCGCTTCTCTGGATCTAGATCTGGGACGGATTTCACTCTGACCATCAGCAGTCTGCAGCCGGAAGACTTCGCAACTTATTACTGTCAGCAACATTATACTACTCCTCCCACGTTCGGACAGGGTACCAAGGTGGAGATCAAA (SEQ ID No.1)
[0066] The protein sequence is shown in SEQ ID No. 2:
[006...
Embodiment 2
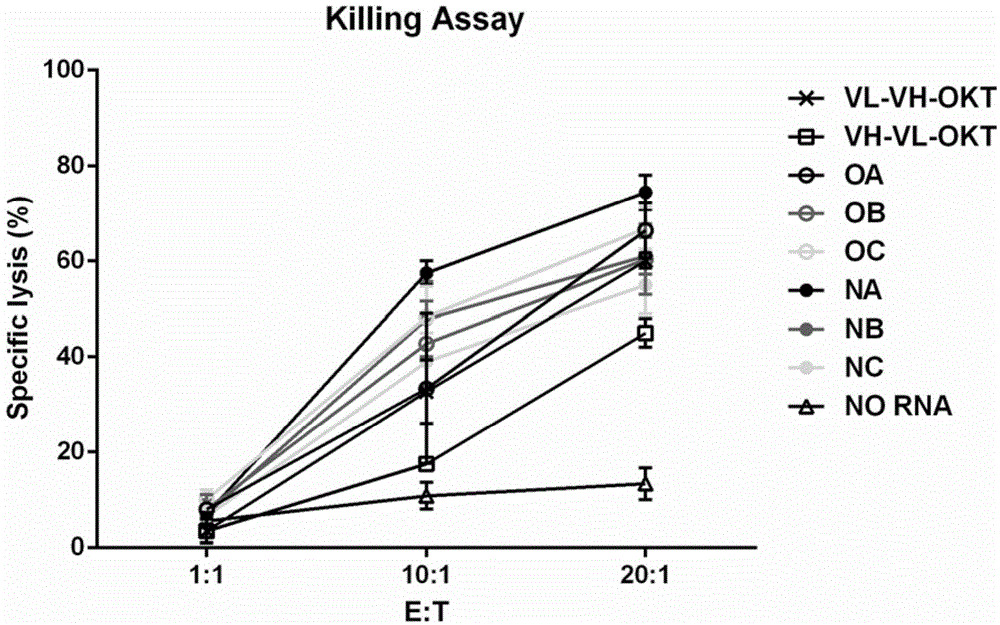
[0141] Based on the above results, we chose the VL-VH method for the 4D5 single-chain structure, and then we further replaced the linker between 4D5VL and 4D5VH, and the linker between 4D5 and OKT. The following six structures were prepared
[0142] (1)OA:VL-(G4S)3-VH-GGGGSDIK-OKT
[0143] The nucleic acid sequence is shown in SEQ ID No. 34:
[0144] ATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAATCAGTGCCTCAGTCATAATGTCCAGAGGAGATATCCAGATGACCCAGTCCCCGAGCTCCCTGTCCGCCTCTGTGGGCGATAGGGTCACCATCACCTGCCGTGCCAGTCAGGATGTGAATACTGCTGTAGCCTGGTATCAACAGAAACCAGGAAAAGCTCCGAAACTACTGATTTACTCGGCATCCTTCCTTTATTCTGGAGTCCCTTCTCGCTTCTCTGGATCTAGATCTGGGACGGATTTCACTCTGACCATCAGCAGTCTGCAGCCGGAAGACTTCGCAACTTATTACTGTCAGCAACATTATACTACTCCTCCCACGTTCGGACAGGGTACCAAGGTGGAGATCAAAggcggagggggaagtggcgggggtgggtccggcggcggcggctcgGAGGTTCAGCTGGTGGAGTCTGGCGGTGGCCTGGTGCAGCCAGGGGGCTCACTCCGTTTGTCCTGTGCAGCTTCTGGCTTCAACATTAAAGACACCTATATACACTGGGTGCGTCAGGCCCCGGGTAAGGGCCTGGAATGGGTTGCAAGGATTTATCCTACGAATGGTTATACTAGATATGCCGATAGCGTCAAGGGCCGTTTCACTATAAGC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com