Pharmaceutical application of agaricin-4-o-β-d-glucoside and its pharmaceutical composition
A technology of larchoresin and glucoside, which is applied in the field of medicine, can solve the problem that there is no relevant literature report on larchoresin-4-O-β-D-glucoside, and achieve the effect of less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
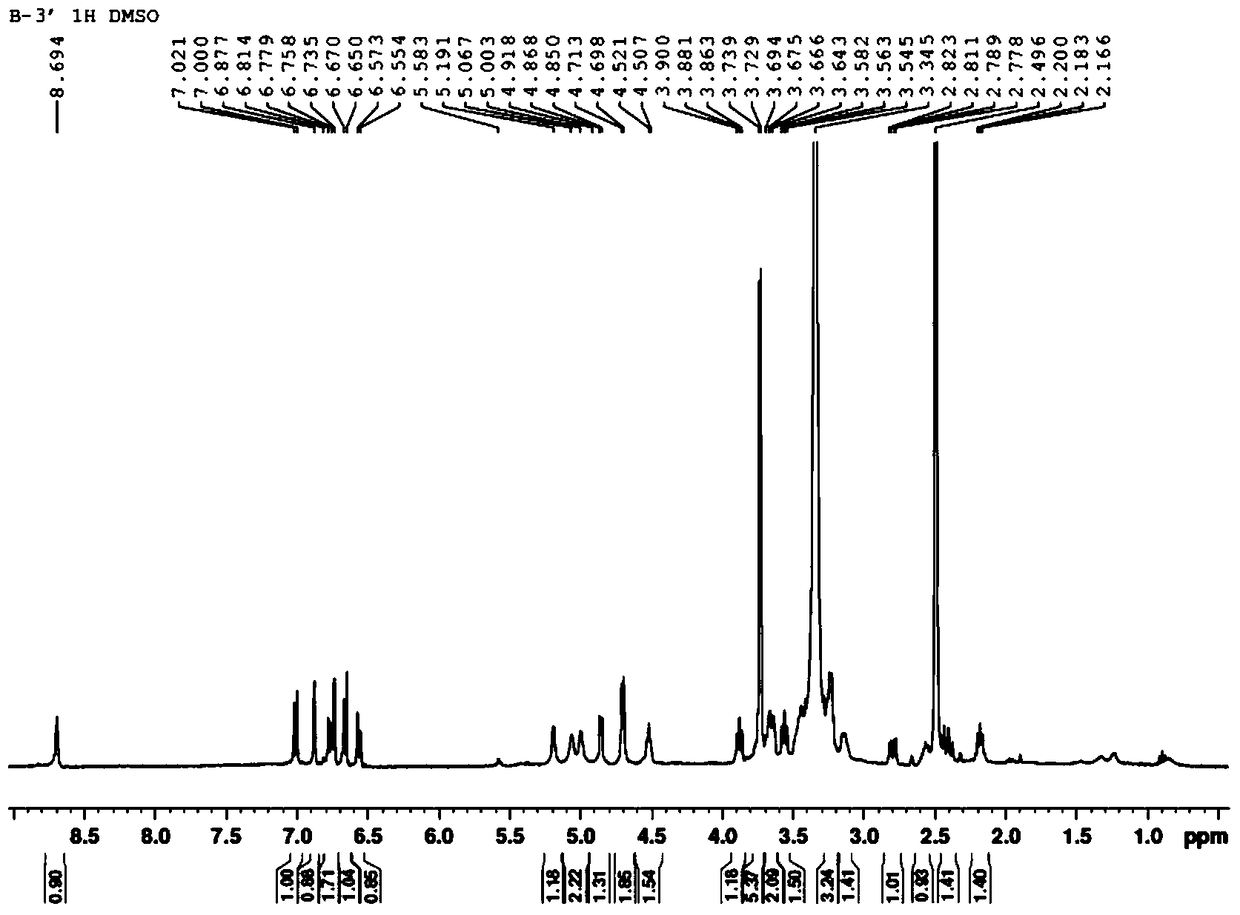
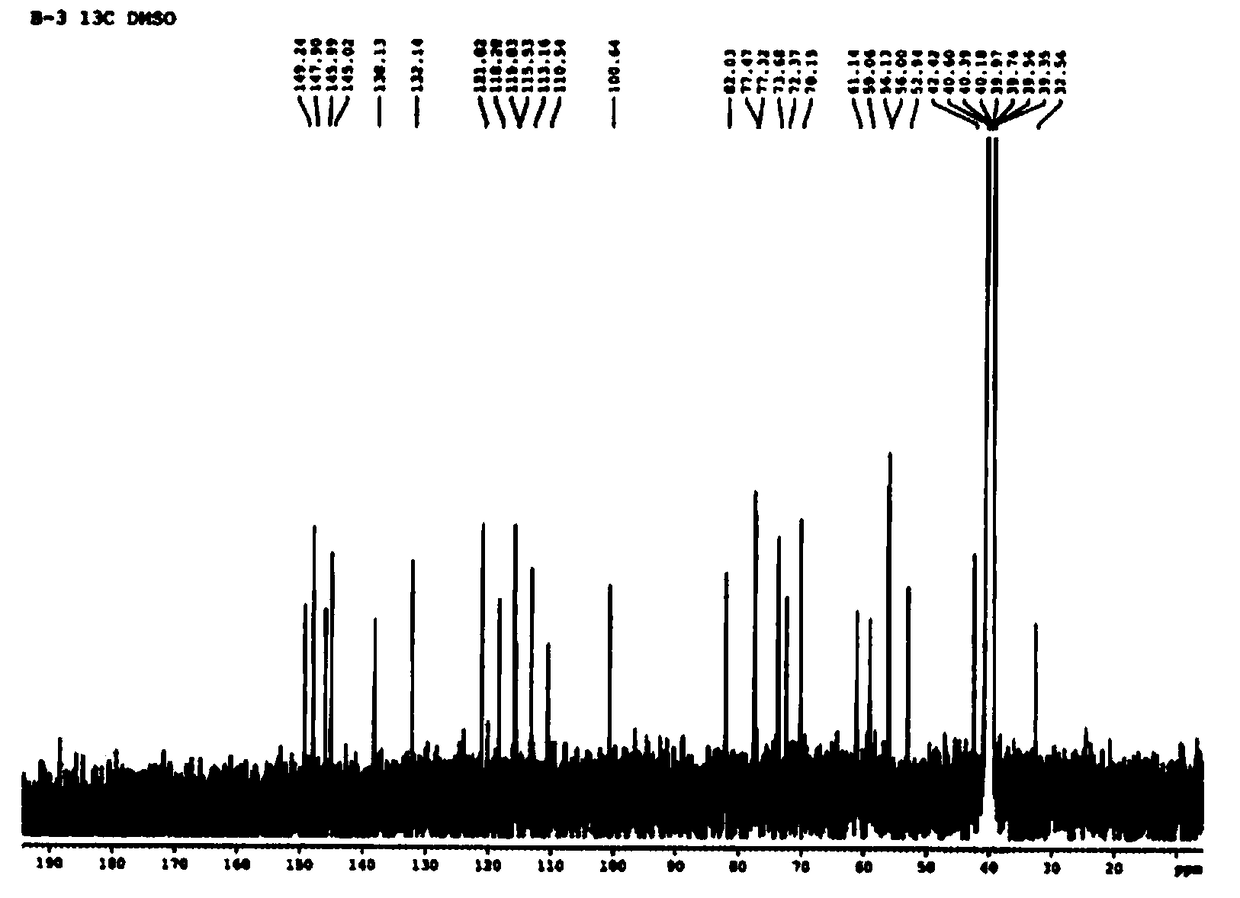
[0027]Example 1 Separation, purification and structural identification of larchoresin-4-O-β-D-glucoside
[0028] According to the existing extraction and separation methods in this field, extract and separate from the Radix Radix Radix Radix methanol extract to obtain larchoresin-4-O-β-D-glucoside, specifically, suspend 500g of Radix Radix Radix Radix methanol extract with water, and then use ethyl acetate, Extraction with n-butanol, 70 g of n-butanol is further desugared by macroporous resin HPD100, and 95% ethanol is collected to recover the solvent to obtain about 45 g of extract. The extract was subjected to silica gel column chromatography (200-300 mesh, 5×30cm), eluted with chloroform-methanol gradient (100:0-98:2-95:5-9:1-2:1-0:1, v / v) 13 components Fr.1-Fr.13 are obtained. Fr.11 was purified by preparative MPLC and eluted with methanol-water gradient (0:100-100:0, v / v) to obtain 7 components A-G. Fr.C was further purified by silica gel column chromatography to obtain ...
Embodiment 2
[0031] Example 2 Preparation of larchoresin-4-O-β-D-glucoside tablet
[0032] Weigh each component according to the following formula:
[0033]
[0034] Tablets were prepared according to the conventional preparation method of tablets in the field of pharmacy, and 1000 tablets were made in total, each containing 5 mg of agaricin-4-O-β-D-glucoside. The usage of this tablet is: 2-4 tablets each time, 3 times a day, seven days as a course of treatment.
Embodiment 3
[0035] Example 3 Preparation of larchoresin-4-O-β-D-glucoside capsules
[0036] Weigh each component according to the following formula:
[0037]
[0038] Prepared according to the conventional method of capsules in the field of pharmacy, a total of 1000 capsules were made, and each capsule contained 5 mg of agaricin-4-O-β-D-glucoside. Usage of the capsule: 2-4 capsules each time, 3 times a day, seven days as a course of treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com