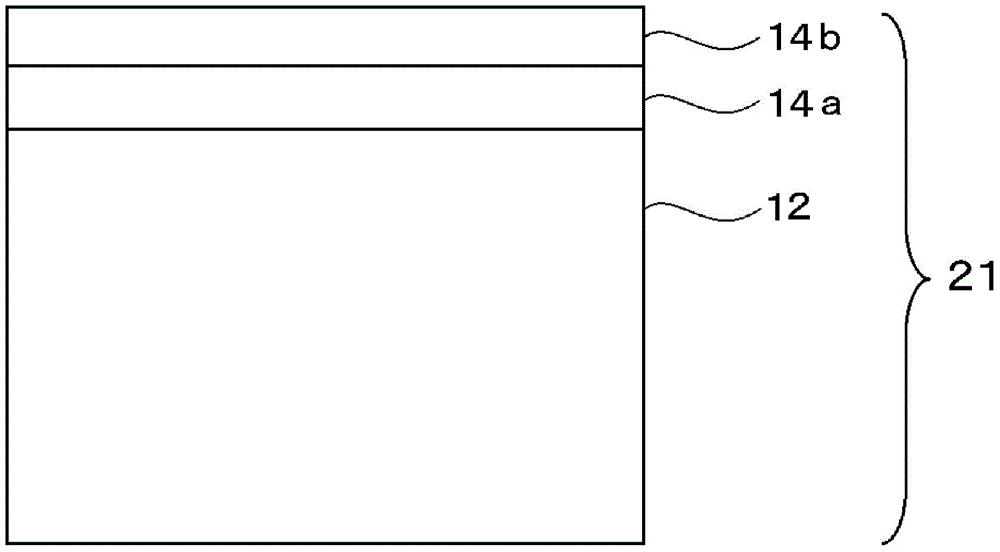
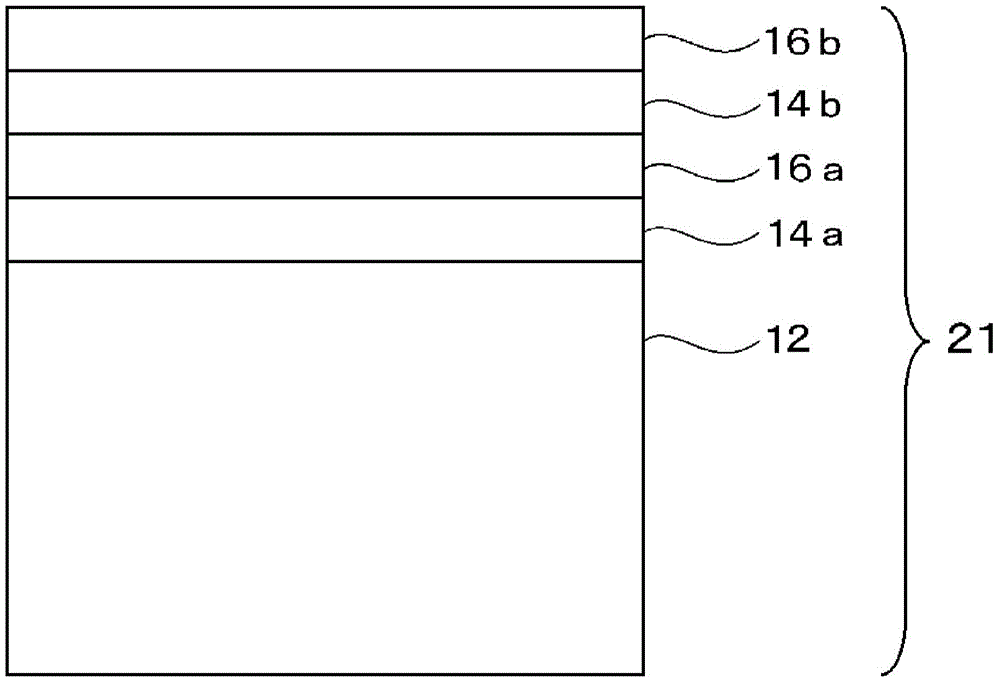
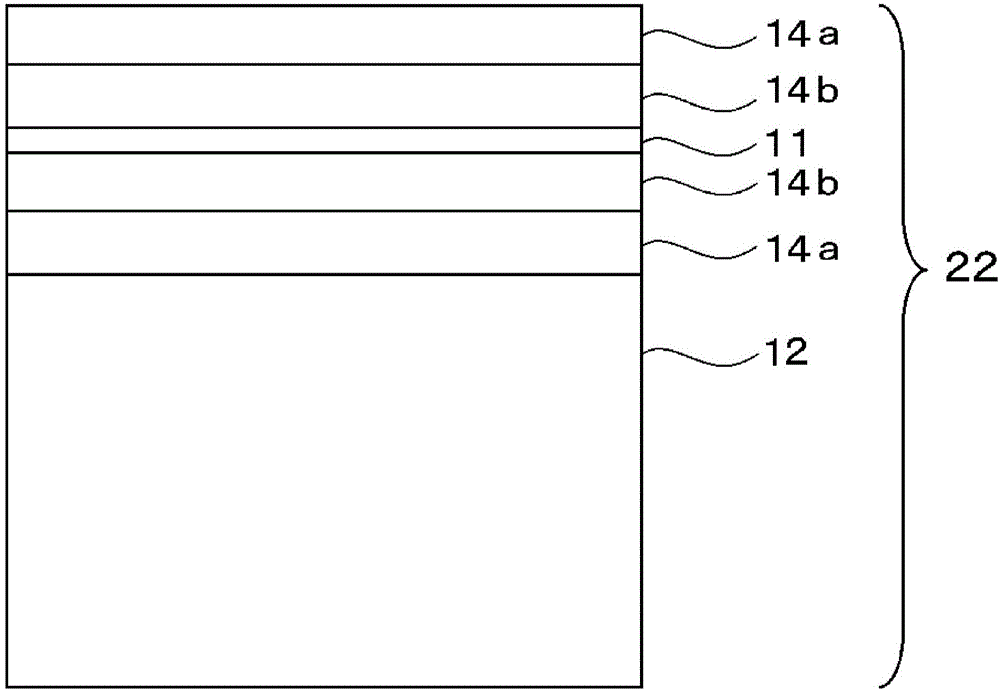
Multilayered cholesteric liquid crystal, process for producing same, and laminate of multilayered cholesteric liquid crystal
A cholesteric and liquid crystal layer technology, which is applied in the field of cholesteric liquid crystal laminates and their manufacture and the combination of cholesteric liquid crystal laminates, can solve the problems of light leakage, poor productivity, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0394]
[0395] The following compound 1, compounds in compound group B, fluorine-based horizontal alignment agent, chiral agent, polymerization initiator, and methyl ethyl ketone as a solvent were mixed to prepare a coating solution with the following composition. The obtained coating liquid was made into the coating liquid (R1) which is a cholesteric liquid crystalline mixture.
[0396]
[0397] ·Compound 1
[0398]
[0399] Compound group B
[0400] ·Compound 2
[0401]
[0402] · Compound 3:
[0403]
[0404] · Compound 4:
[0405]
[0406] Fluorine horizontal alignment agent 1
[0407]
[0408] Fluorine horizontal alignment agent 2
[0409]
[0410]
[0411]
[0412] The above-mentioned compound 1, the compound in the above-mentioned compound group B, a fluorine-based horizontal alignment agent, a chiral agent, a polymerization initiator, and methyl ethyl ketone as a solvent were mixed to prepare a coating liquid having the following compo...
Embodiment 2
[0446]
[0447] The following compound 1, compounds in compound group B, fluorine-based horizontal alignment agent, chiral agent, polymerization initiator, and methyl ethyl ketone as a solvent were mixed to prepare a coating solution with the following composition. The obtained coating liquid was made into the coating liquid (L1-2) which is a cholesteric liquid crystalline mixture.
[0448]
[0449]
[0450] Left-handed chiral agent (B)
[0451]
[0452]
[0453] Except for using the coating liquid (L1-2) instead of the coating liquid (L1-1), the cholesteric liquid crystal phase was fixed on the film (F1) prepared in Example 1 by the same method as in Example 1 to prepare A cholesteric liquid crystal laminate (G1-2) in which two layers of cholesteric liquid crystal phases were fixed on a PET film was prepared. The produced cholesteric liquid crystal laminate (G1-2) had no remarkable defects and streaks, and had a good surface state. This cholesteric liquid cryst...
Embodiment 3
[0457]
[0458] The following compound 1, compounds in compound group B, fluorine-based horizontal alignment agent, chiral agent, polymerization initiator, and methyl ethyl ketone as a solvent were mixed to prepare a coating solution with the following composition. The obtained coating liquid was made into the coating liquid (L1-3) which is a cholesteric liquid crystalline mixture.
[0459]
[0460]
[0461] Left-handed chiral agent (C)
[0462]
[0463]
[0464] Except for using the coating liquid (L1-3) instead of the coating liquid (L1-1), the cholesteric liquid crystal phase was immobilized on the film (F1) produced in Example 1 by the same method as in Example 1 to prepare A cholesteric liquid crystal laminate (G1-3) in which two layers of cholesteric liquid crystal phases were fixed on a PET film was prepared. The produced cholesteric liquid crystal laminate (G1-3) had no remarkable defects and streaks, and had a good surface state. This cholesteric liquid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com