Spermidine/spermine n1-acetyltransferase substrates as anti-cancer drug compounds
A technology of acetyltransferase and anticancer drugs, which is applied in the field of spermidine/spermine N1-acetyltransferase substrates used as anticancer drug compounds, and can solve problems such as increased enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The invention discloses a method of combining spermidine / spermine N 1 - Methods of acetyltransferase (SSAT) substrates as anticancer drug compounds.
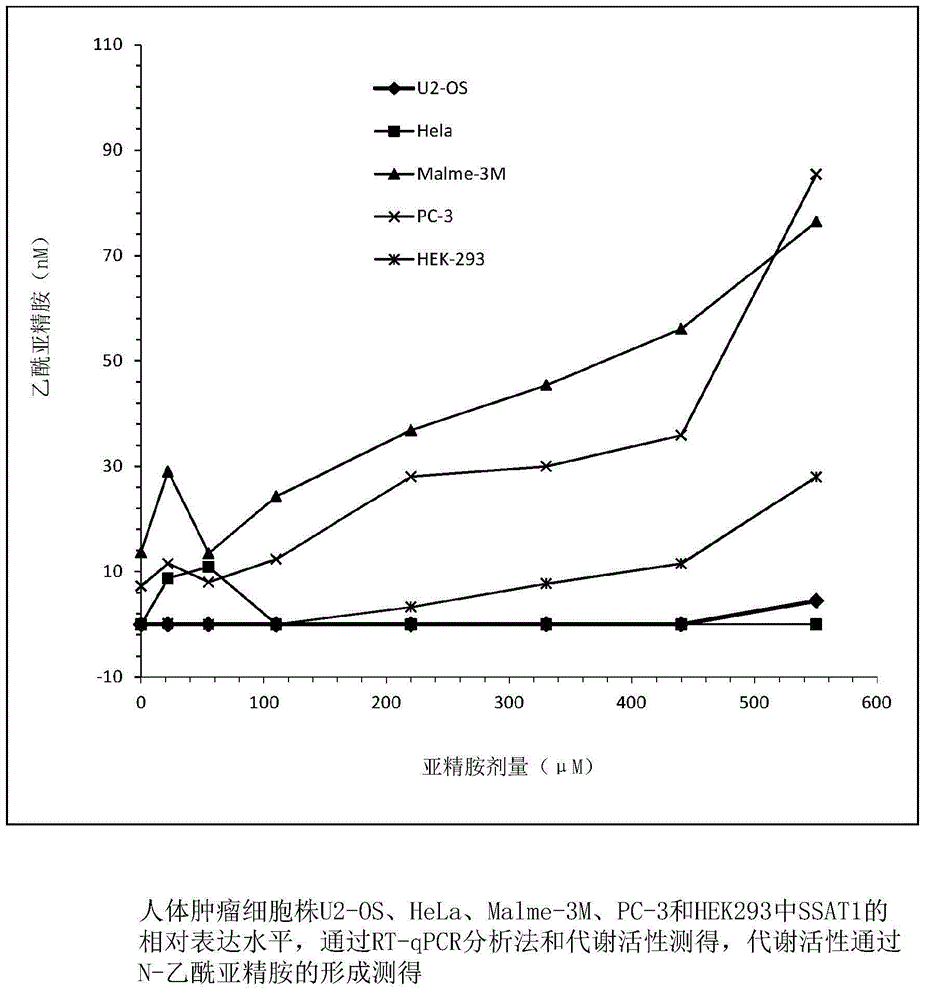
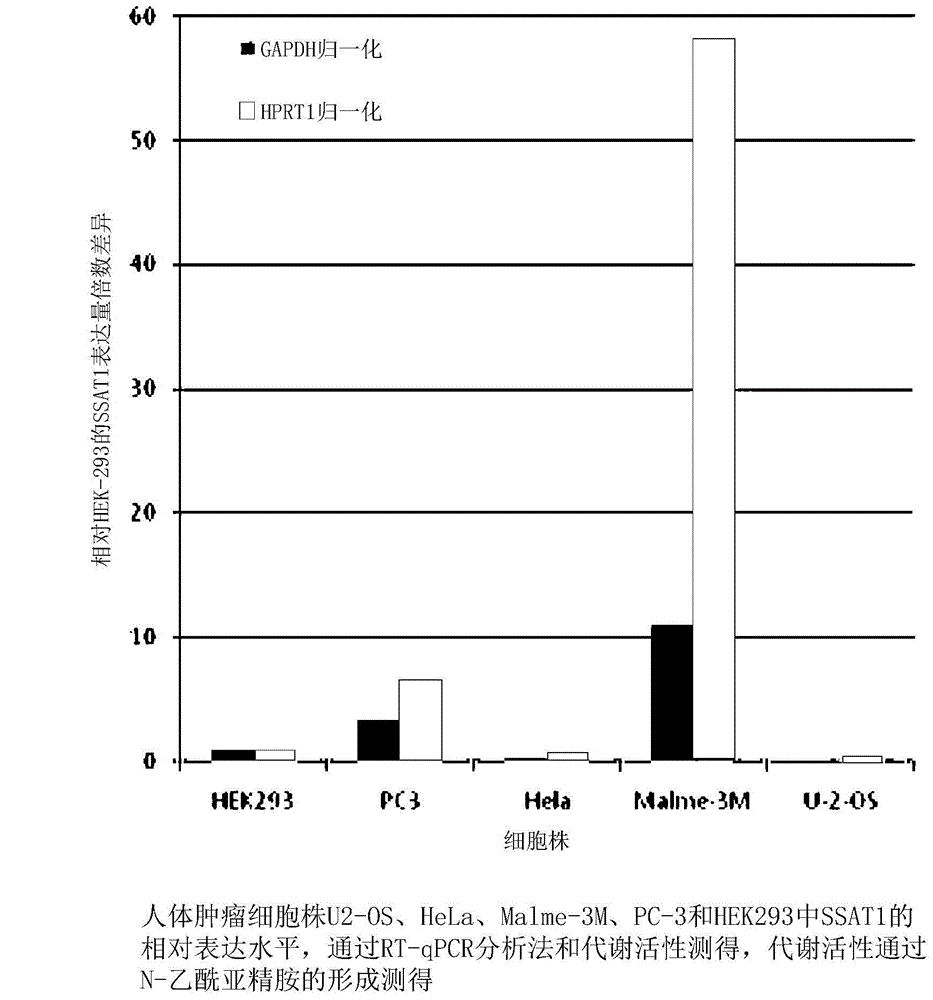
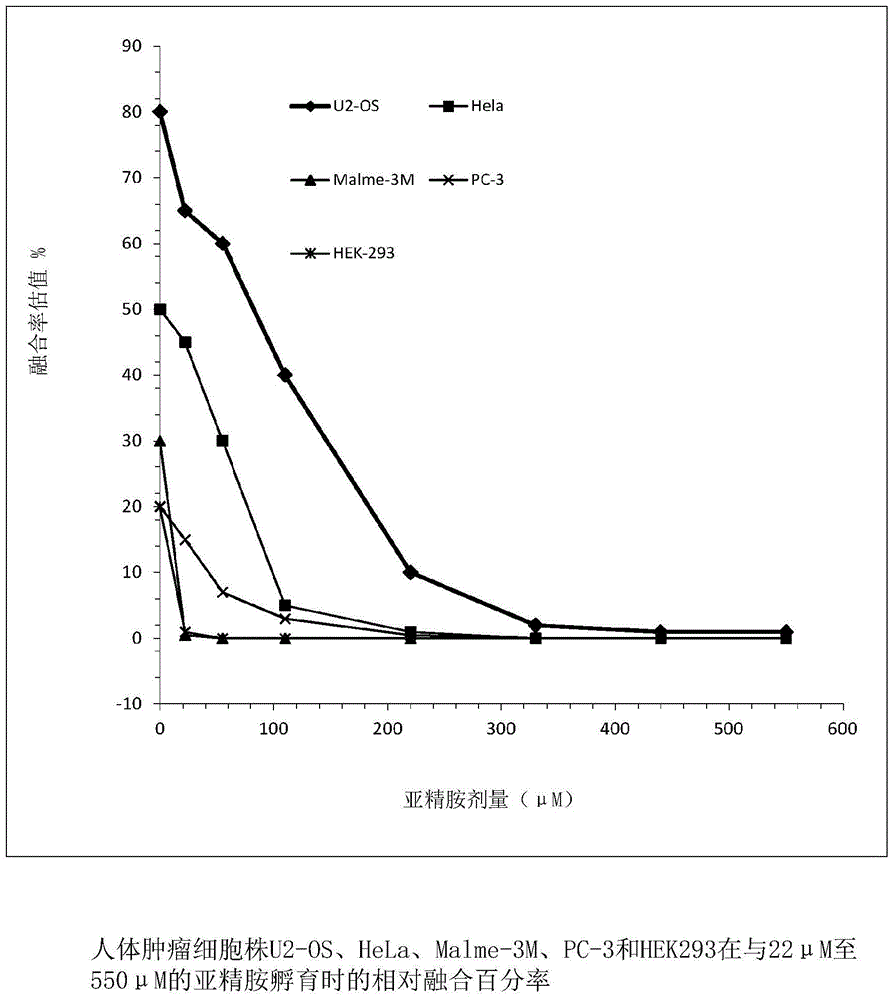
[0027] Relative expression levels of SSAT in human tumor cell lines HEK-293, Malme-3M, HeLa, PC-3 and US-02 were determined by reverse transcription-quantitative polymerase chain reaction analysis (RT-qPCR analysis), and Such as figure 1 with figure 2 As shown, it was observed that the relative expression of SSAT in Malme-3M was the highest. When normalized by GAPDH, the relative expression of SSAT in Malme-3M was 11 times higher than that of the control HEK-293 cell line. When normalized by HPRT1, The relative expression of SSAT in Malme-3M was 58 times higher than that in the control HEK-293 cell line. The relative expression level of SSAT in PC-3 was the second highest, and when normalized by GAPDH and HPRT1, the relative expression level of SSAT in PC-3 was about 3-fold and 7-fold different from HEK-293, respectiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com