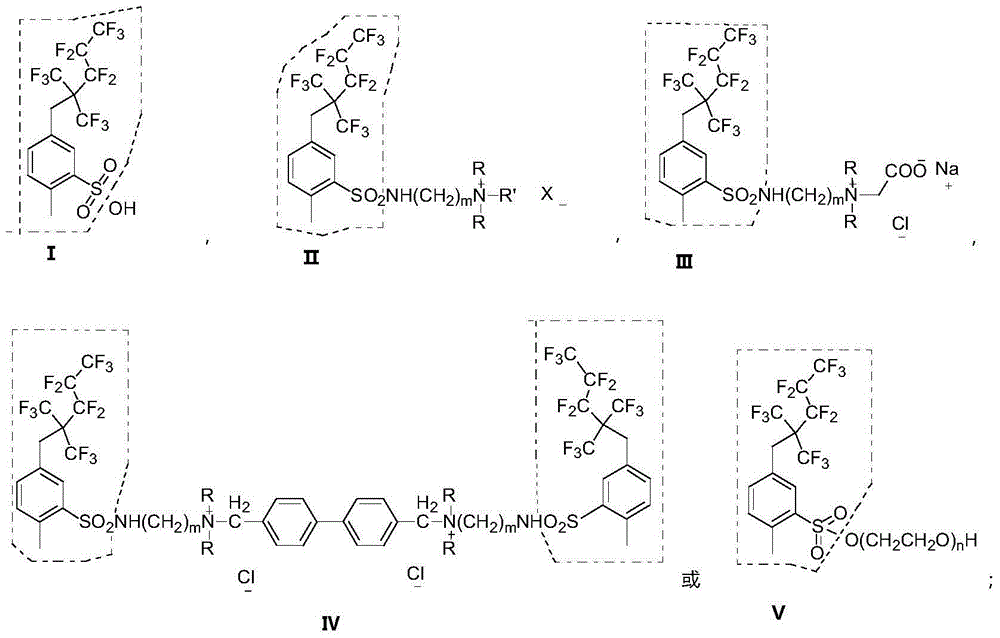
Branch fluorocarbon chain containing fluorocarbon surfactant and preparation method thereof
A technology of fluorocarbon surface and active agent, which is applied in the preparation of sulfonic acid, sulfonate ester, sulfonamide, etc., and can solve problems such as less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
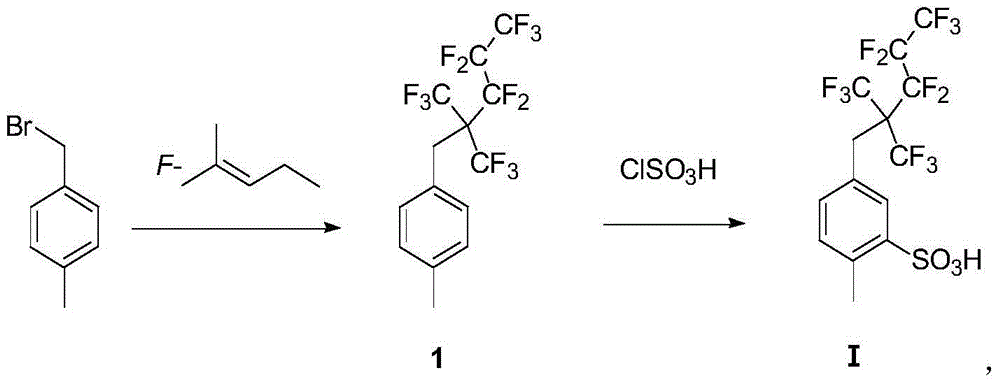
[0054] Embodiment 1: the synthesis of fluorine-containing intermediate amide compound 6
[0055]
[0056] 1.84g (10mmol, 1equiv) 4-methylbenzyl bromide, 1.16g (20mmol, 2equiv) potassium fluoride, 20mL DMF and 4.5g (15mmol, 1.5equiv) perfluoro-2-methyl-2 - Pentene. The system was reacted at 80°C for 10h. The system was poured into water, extracted with petroleum ether, the combined PE extracts were washed successively with saturated sodium bicarbonate and saturated brine, then dried over anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure. The crude product was passed through a PE column to obtain 3.3g Colorless liquid compound 1, yield 77.8%.
[0057] 1 H NMR (CDCl 3 ,300MHz): δ(ppm)2.36(s,CH 3 ,3H),3.53(s,CH 2 ,2H),7.15-7.17(d,J=8.1Hz,Ar-H,2H),7.17-7.24(d,J=8.1Hz,Ar-H,2H); 19 F NMR (CDCl 3 ,282MHz,):δ(ppm)-61.05~-61.25(m,6F),-78.85~-78.98(t,3F),-104.60~-104.95(m,2F),-121.65~-121.90(m, 2F); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 20...
Embodiment 2
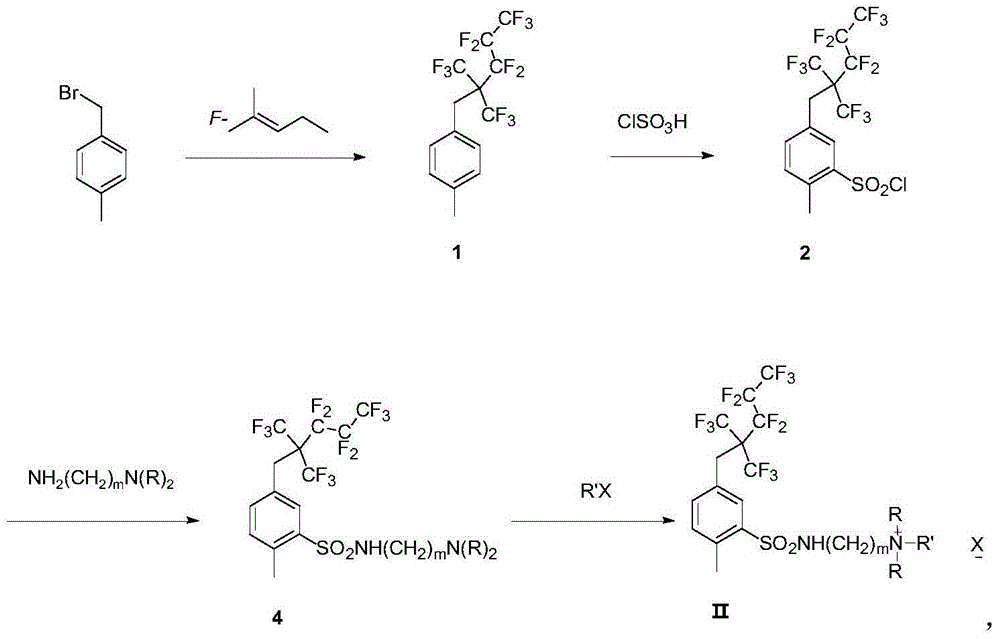
[0062] Example 2: Synthesis of quaternary ammonium salt cationic fluorocarbon surfactants containing branched fluorocarbon chains
[0063]
[0064] 0.287g (0.5mmol, 1equiv) of compound 6, 0.156g (1mmol, 2equiv) of iodoethane and 1mL of acetonitrile were reacted at 80°C for 5h in a 5mL sealed tube under the protection of argon. The system was lowered to room temperature, and the solvent was removed by rotary evaporation under reduced pressure, and the crude product was subjected to CH 2 Cl 2 :methanol=9:1 column to obtain 200mg white powder compound.
[0065] 1 H NMR (DMSO-d 6 ,300MHz): δ(ppm)1.15-1.25(t, J=6.6Hz, N(CH 3 ) 2 CH 2 CH 3 ,3H),2.57(s,CH 3 ,3H),3.02(s,N(CH 3 ) 2 ,6H),3.06-3.16(q,N(CH 3 ) 2 CH 2 CH 3 ,2H),3.30~3.36(m,NHCH 2 CH 2 N(CH 3 ) 2 CH 2 CH 3 ,4H),3.85(s,C 6 f 13 CH 2 C 6 h 3 ,2H),7.42-7.48(d,J=8.1Hz,Ar-H,1H),7.48-7.55(d,J=8.1Hz,Ar-H,1H),7.84(s,Ar-H,1H) ,8.08-8.15(t,J=5.7Hz,NH,1H); 19 F NMR (DMSO-d 6 ,282MHz,):δ(ppm)-61.95~-62.14...
Embodiment 3
[0066] Example 3: Synthesis of betaine-type amphoteric fluorocarbon surfactants containing branched fluorocarbon chains
[0067]
[0068] 0.316g (0.55mmol, 1.1equiv) of compound 6, 0.058g (0.5mmol, 1equiv) of sodium chloroacetate and 1mL of acetonitrile were reacted at 80°C for 12h in a 5mL sealed tube under argon protection. The system was filtered, and the solid was first washed with acetonitrile, then washed with acetone, then dissolved in methanol, filtered, and the solvent was removed from the filtrate to obtain 0.178 g of a white solid compound.
[0069] 1 H NMR (CD 3 OD,300MHz)δ:2.60(s,C 6 h 3 CH 3 ,3H),3.20~3.30(m,8H),3.70~3.85(m,6H),7.37(d,J=7.8Hz,Ar-H,1H),7.48(d,J=7.8Hz,Ar-H ,1H),7.92(s,Ar-H,1H); 19 F NMR (CD 3 OD,282MHz)δ:-63.60~-63.85(m,6F),-81.94(t,J=13.7Hz,3F),-106.90~-107.30(m,2F),-124.00~-124.30(m,2F ); 13 C NMR (100MHz, CD 3 OD): δ (ppm) 19.7, 37.9, 52.4, 62.6, 63.5, 65.5, 130.7, 133.4, 133.7, 137.1, 138.5, 138.6, 168.2; LRMS (MALDI): 655.0 (M-Cl)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap