Application of teicoplanin in the preparation of anti-Middle East respiratory syndrome coronavirus drugs
A technology for respiratory syndrome and teicoplanin, applied in the direction of antiviral agents, glycopeptide components, etc., can solve the problems of no antiviral drugs, lack of vaccines and drugs, etc., and achieve the effect of avoiding a long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
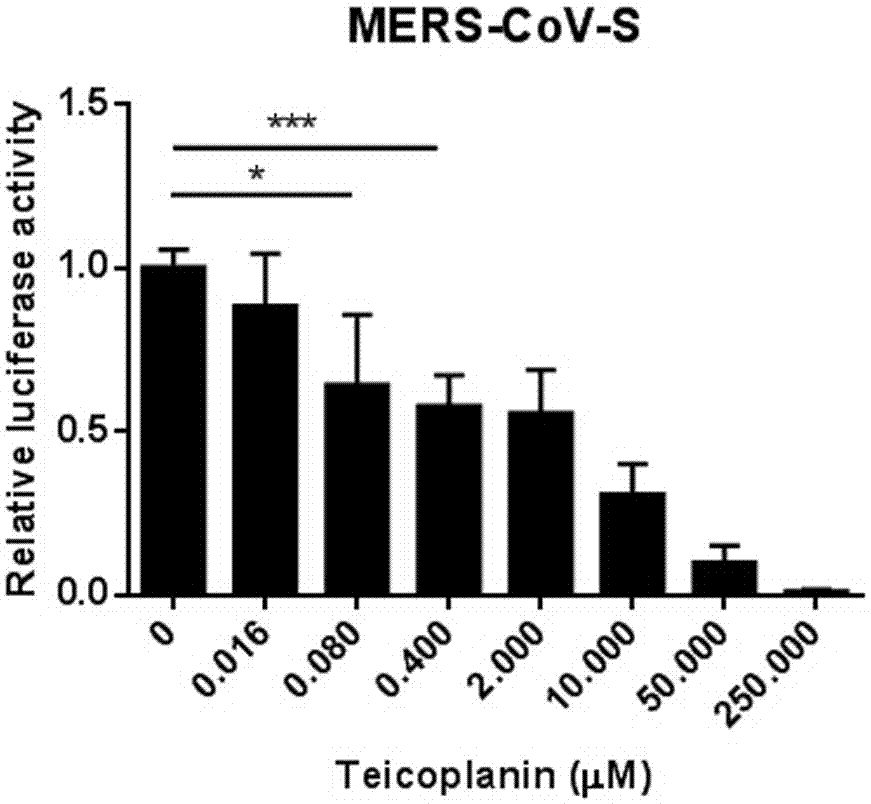
[0037] Example 1 The inhibitory effect of the compound antibiotic teicoplanin at different concentrations on the envelope protein S of Middle East respiratory syndrome coronavirus (MERS-CoV)
[0038] (1) Encapsulate virus: 4.5 μg pHIV-luciferase, 4.5 μg pCMV-ΔR8.2 and 10 μg MERS-CoV-S plasmids were transfected into 293T cells (10 cm dish). After 48 hours, the virus supernatant was collected and tested. p24.
[0039] (2) Compound screening for MERS-CoV-S: p24-normalized MERS-CoV-S pseudovirus supernatant (containing 8 μg / ml Polybrene) was used to infect 293T cells in a 96-well plate, and at the same time, different concentrations of substitute test were added Lanin compound.
[0040] (3) Change the medium: After 12 hours of infection, change to fresh DMEM medium.
[0041] (4) Detection of luciferase activity: 48 hours after infection, wash each well with PBS once, then add 100 μl lysate, shake for 30 min, and take 10 μl lysate to detect luciferase activity.
[0042] According to the re...
Embodiment 2
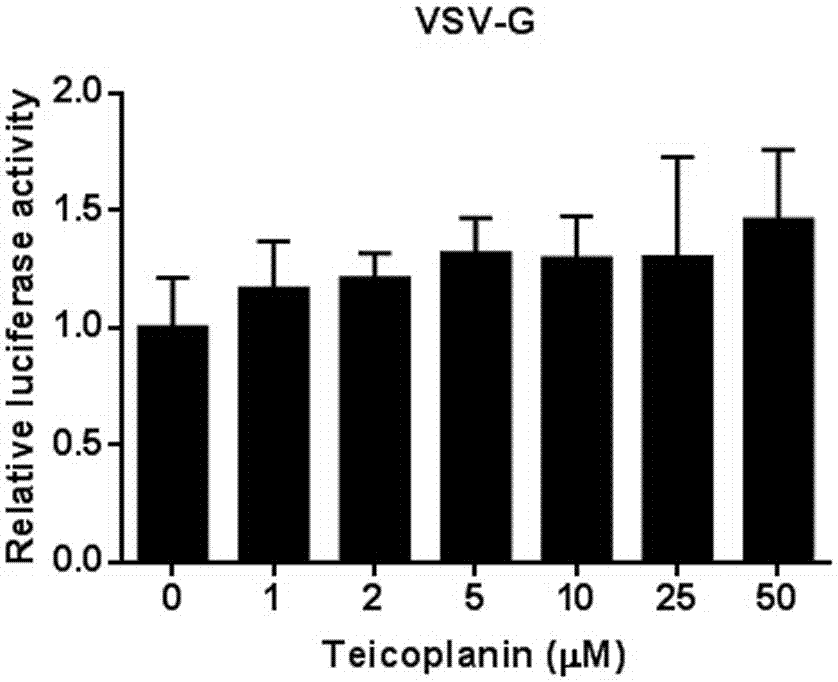
[0043] Example 2 The inhibitory effect of the compound antibiotic teicoplanin at different concentrations on the vesicular stomatitis virus envelope VSV-G
[0044] (1) Encapsulate virus: 4.5 μg pHIV-luciferase, 4.5 μg pCMV-ΔR8.2 and 10 μg VSV-G plasmids were transfected into 293T cells (10 cm dish). After 48 hours, the virus supernatant was collected and p24 was measured.
[0045] (2) Compound screening for VSV-G: p24-normalized VSV-G pseudovirus supernatant (containing 8 μg / ml Polybrene) was infected with 293T cells in a 96-well plate, and teicoplanin compounds of different concentrations were added at the same time.
[0046] (3) Change the medium: 12 hours after infection, change to fresh DMEM medium.
[0047] (4) Detection of luciferase activity: 48 hours after infection, wash each well with PBS once, then add 100 ul lysate, shake for 30 minutes, take 10 ul lysate to detect luciferase activity.
[0048] According to the result of Example 2 figure 2 It shows that the antibiotic teico...
Embodiment 3
[0049] Example 3 The inhibitory effect of the compound antibiotic teicoplanin at different concentrations on the envelope protein S of severe acute respiratory syndrome coronavirus (SARS-CoV)
[0050] (1) Encapsulate virus: 6.5 μg pHIV-luciferase, 8 μg pCMV-ΔR8.2 and 20 μg SARS-CoV-S plasmids were transfected into 293T cells (10 cm dish). After 48 hours, the virus supernatant was collected and tested. p24.
[0051] (2) Compound screening for SARS-CoV-S: p24-normalized SARS-CoV-S pseudovirus supernatant (containing 8 μg / ml Polybrene) was infected with 293T cells in 96-well plates, and at the same time added different concentrations of substitute test Lanin compound.
[0052] (3) Change the medium: After 12 hours of infection, change to fresh DMEM medium.
[0053] (4) Detection of luciferase activity: 48 hours after infection, wash each well with PBS once, then add 100 μl of lysate, shake for 30 min, and take 10 μl of lysate to detect luciferase activity.
[0054] According to the resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com