Prodrug based on gemcitabine structure and application thereof
A technology of gemcitabine and prodrugs, applied in the field of nucleoside drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] Below in conjunction with the accompanying drawings and examples, the specific implementation of the present invention will be further described in detail, the following examples are used to illustrate the present invention, but not to limit the scope of the present invention.
[0035] 1. Synthesis and preparation of prodrugs based on gemcitabine structure.
[0036] The general structural formula of the prodrug based on the gemcitabine structure claimed in the present invention is shown in formula (I):
[0037]
[0038] where R is CH 2 or And m is an odd number of 13-17, and n is 0 or 1.
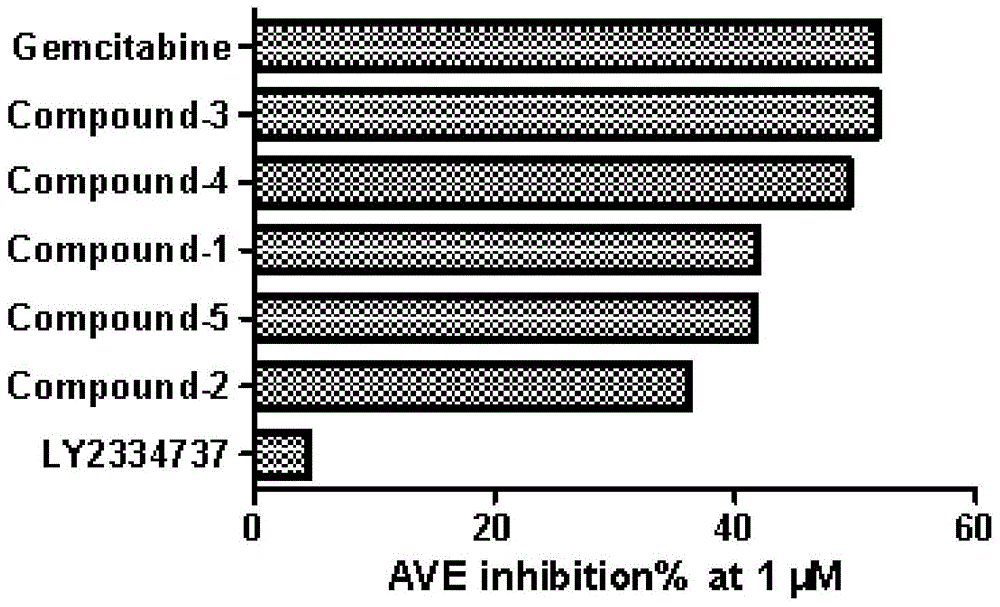
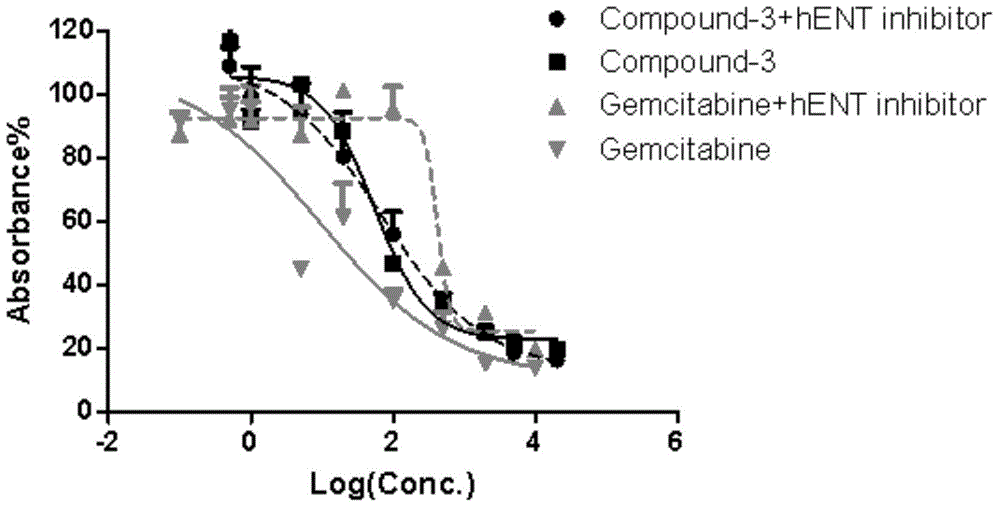
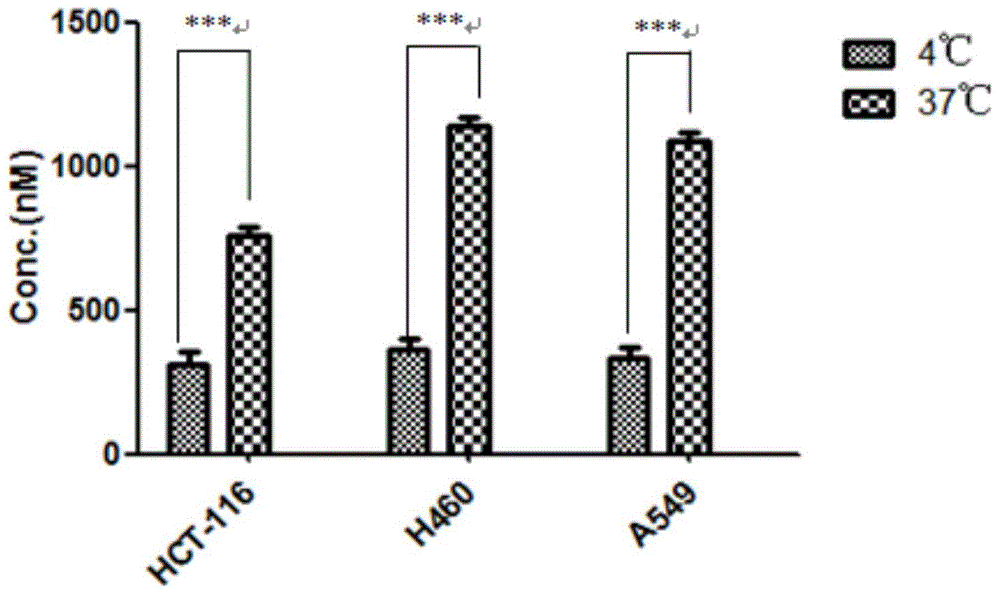
[0039] Five kinds of prodrugs conforming to the general formula are disclosed in this specific example, named respectively as compound-1, compound-2, compound-3, compound-4 and compound-5, the structural formulas of these five kinds of prodrugs and The preparation method is as follows.
[0040] Preparation of N-acetyl-4-acetyl-gemcitabine: Gemcitabine (3g, 10mmol) was dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com