Application of (Z)-2-imino-5-(3,5-dimethoxyphenylmethylene)-1-methylimidazolidinyl-4-one in preparation of cardiovascular drugs
A kind of dimethoxybenzyl, cardiovascular technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of (Z)-2-imino-5-(3,5-dimethoxybenzylidene)-1-methylimidazolidin-4-one (compound IV5).
[0022]
[0023] 3,5-Dimethoxybenzaldehyde (0.83g, 5mmol), creatinine (0.57g, 5mmol), molten sodium acetate (2.05g, 25mmol) and glacial acetic acid (7mL) were stirred under reflux for 10h, cooled After reaching room temperature, 5 mL of water was added, suction filtered, washed with water, and recrystallized with DMF and water to obtain 1.09 g of dark yellow-brown crystals (Compound IV5), with a yield of 84.2%. The physical and chemical identification results are as follows:
[0024] mp: 222~223°C;
[0025] IR (KBr, cm -1 ): 3287, 2999, 2842, 1703, 1660, 1588, 1558, 1505, 1458;
[0026] 1 H-NMR (400MHz, DMSO-d 6 ): δ (ppm) 3.17 (s, 3H, CH 3 ), 3.79 (s, 6H, CH 3 ), 6.139 (s, 1H, =CH), 6.216 (s, 1H, Ar), 7.58 (s, 2H, ArH);
[0027] HR-MS: C 13 h 15 N 3 o 3 Theoretical value: 261.1113, actual value: 261.1111.
Embodiment 2
[0028] Example 2: The protective effect of compound IV5 on primary cardiomyocytes cultured in vitro against glucose-hypoxia / reperfusion injury.
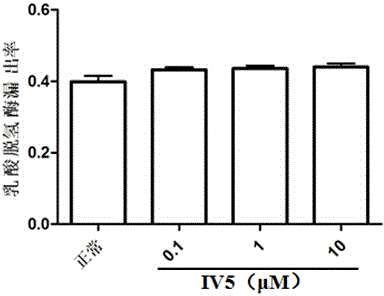
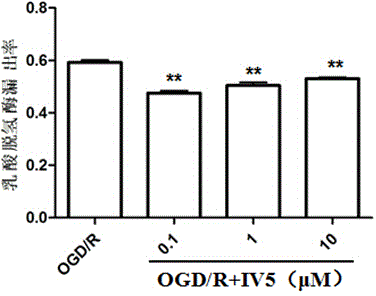
[0029] Rat primary cardiomyocytes were cultured for 72 hours, and randomly divided into non-glucose hypoxia / reoxygenation control group ( figure 1 denoted as "normal" in the group), non-glucose hypoxia / reoxygenation + IV5 group ( figure 1 expressed as "IV5" in , where the doses of IV5 were 0.1, 1, and 10 μM), the glucose-deficient hypoxia / re-oxygenation group ( figure 2 denoted as "OGD / R") and glucose hypoxia / reoxygenation+IV5 group ( figure 2 Expressed as "OGD / R+IV5", where the dosage of IV5 is 0.1, 1, 10 μM respectively). Treat with hypoxia and glucose for 3 hours, then replace the medium with normal glucose and reoxygenate for 12 hours, use the LDH method to detect the degree of cell damage, and calculate the leakage rate of lactate dehydrogenase (LDH) according to the following formula (%) = A 培养液 / (A 培养液 +A 细胞均浆液 ) × 100%...
Embodiment 3
[0032] Example 3: Protective effect of compound IV5 on myocardial ischemia / reperfusion injury in rats.
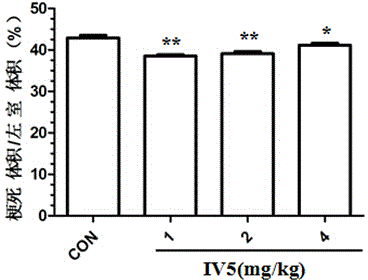
[0033] Male SD rats were randomly divided into control group (sham operation group, image 3 Indicated as "CON"), model group, high-, medium-, and low-dose IV5 groups (the dosage of IV5 was 4 mg / kg, 2 mg / kg, and 1 mg / kg, respectively), 10 rats in each group. Myocardial ischemia model in rats was made by ligating the left anterior descending coronary artery. After 30 minutes of ischemia, the rats were reperfused. Immediately after reperfusion, IV5 was injected into the common jugular vein with different doses and equal volumes. The effect of IV5 on left ventricular infarct volume was observed by TTC staining.
[0034] image 3 shows the effect of compound IV5 on reducing the infarct volume of rat left ventricle (mean±SD, n=10; compared with model group, **p<0.01). It can be known that compound IV5 has a protective effect on myocardial ischemia / reperfusion injury in rats.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com