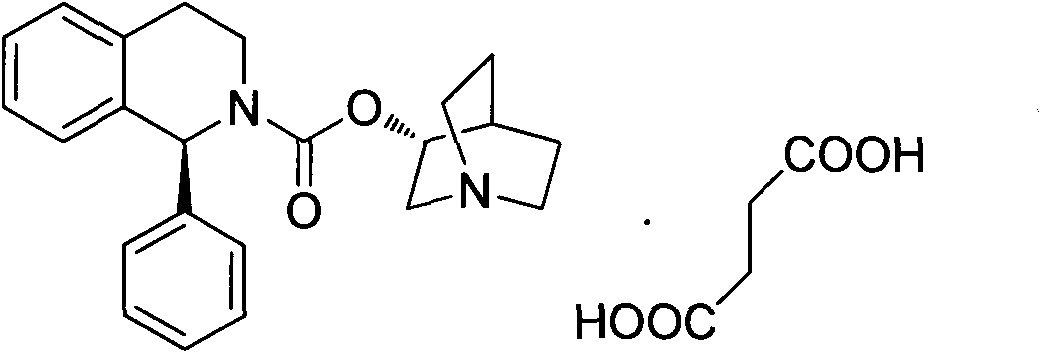
Stable pharmaceutical composition and its preparation method
A technology for compositions and medicines, applied in the directions of medicine combinations, medicine formulations, active ingredients of heterocyclic compounds, etc., can solve the problems of difficult control of results, great difficulty, complicated operation, etc., and achieves reduction of related substances, low equipment requirements, and stability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Tablet core prescription (calculated per 100,000 tablets):
[0035]
[0036] Tablet core: pass solifenacin succinate through a 100-mesh sieve, and other excipients through a 50-mesh sieve; weigh the raw and auxiliary materials of the prescribed amount; put the raw and auxiliary materials except magnesium stearate in a SYH-50 three-dimensional mixer , close the feed port of the mixer, and the pre-mixing time is 30 minutes; open the feed port of the three-dimensional mixer, add the weighed magnesium stearate into the SYH-50 three-dimensional mixer, and close the feed port of the mixer , the mixing time is 2 minutes; tablet compression is carried out after the total mixing is completed. During the production process, samples should be taken to check the tablet appearance, hardness, friability and average tablet weight and record them.
[0037] Coating: Weigh the prescribed amount of Opadry, dissolve it in purified water at a ratio of 12% (w / w), and stir for 30 minutes t...
Embodiment 2
[0040] Tablet core prescription (calculated per 100,000 tablets):
[0041]
[0042] Tablet core: pass solifenacin succinate through a 100-mesh sieve, and other excipients through a 50-mesh sieve; weigh the raw and auxiliary materials of the prescribed amount; put the raw and auxiliary materials except magnesium stearate in a SYH-50 three-dimensional mixer , close the feed port of the mixer, and the pre-mixing time is 30 minutes; open the feed port of the three-dimensional mixer, add the weighed magnesium stearate into the SYH-50 three-dimensional mixer, and close the feed port of the mixer , the total mixing time is 2 minutes; after the mixing is completed, tablet compression should be carried out. During the production process, samples should be taken to check the tablet appearance, hardness, friability and average tablet weight, and record them.
[0043] Coating: Weigh the prescribed amount of Opadry, dissolve it in purified water at a ratio of 12% (w / w), and stir for 30 ...
Embodiment 3
[0046] Tablet core prescription (calculated per 100,000 tablets):
[0047]
[0048]
[0049] Tablet core: pass solifenacin succinate through a 100-mesh sieve, and other auxiliary materials through a 50-mesh sieve; weigh the raw and auxiliary materials of the prescribed amount; put the raw and auxiliary materials except silicon dioxide in a SYH-50 three-dimensional mixer, Close the feed port of the mixer, and the pre-mixing time is 30 minutes; open the feed port of the three-dimensional mixer, add the weighed silicon dioxide in the SYH-50 type three-dimensional mixer, close the feed port of the mixer, the total The mixing time is 5 minutes; tablet compression is carried out after the total mixing is completed. During the production process, samples should be taken to check the tablet appearance, hardness, friability and average tablet weight and record them.
[0050] Coating: Weigh the prescribed amount of Opadry, dissolve it in purified water at a ratio of 12% (w / w), and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com