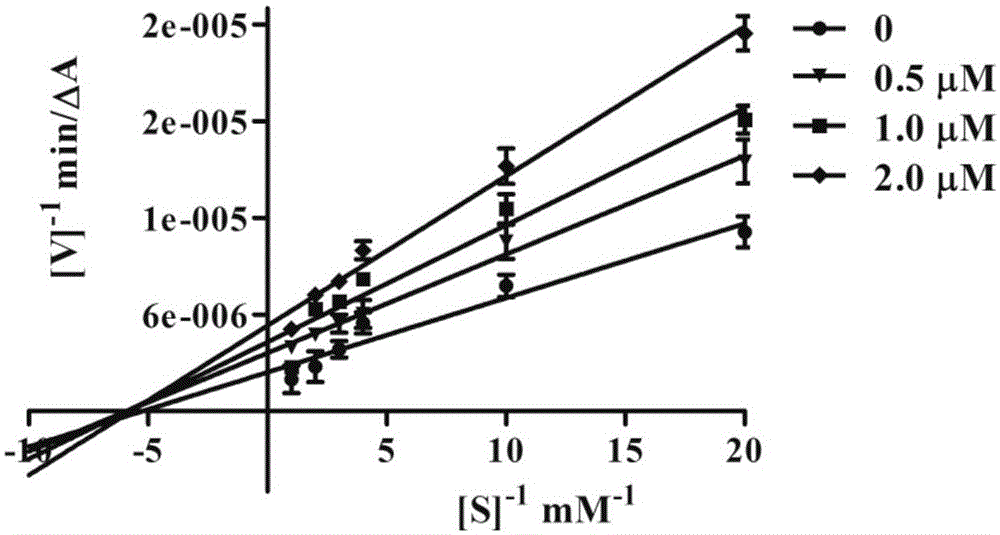
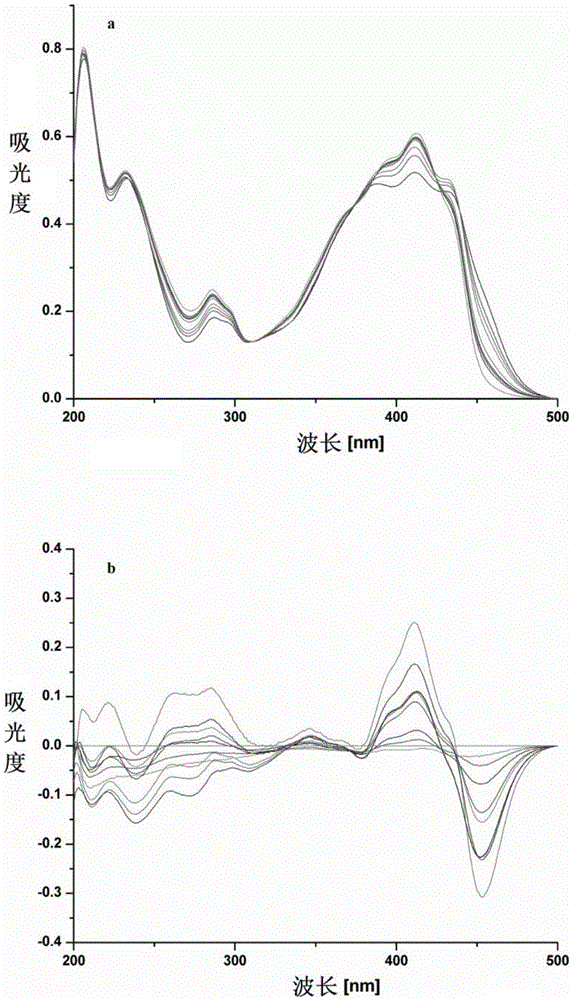
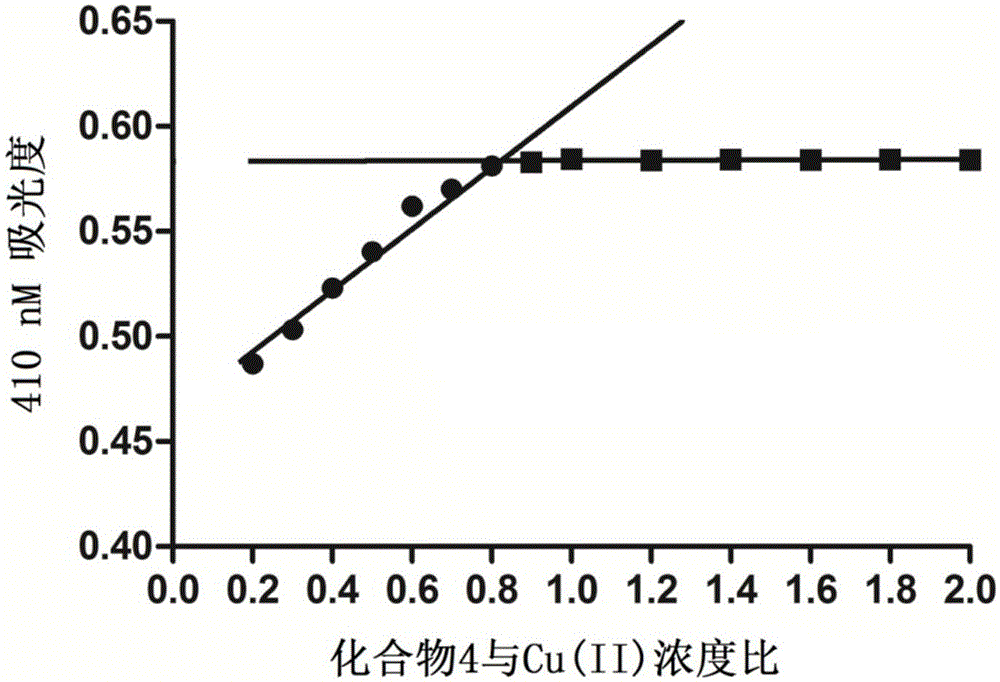
4-hydroxycoumarin-3-Schiff base derivate and use thereof for curing Alzheimer's disease
A technology of hydroxycoumarin and Schiff base, applied in the direction of drug combination, active ingredients of heterocyclic compounds, nervous system diseases, etc., can solve the problem of inhibiting the pathological process of AD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of intermediate 3-amino-4-hydroxycoumarin (1b)
[0058] 3.0g of raw material 3-nitro-4-hydroxycoumarin (1a) was dissolved in 200ml of absolute ethanol, and a catalytic amount of 10% Pt / C was added, and in H 2 Under the atmosphere, react at room temperature for 6-8 hours. After monitoring the reaction, remove Pt / C by suction filtration, and recrystallize from ethanol to obtain about 2.1 g of white solid 1b, with a yield of about 70%. m.p.220-222℃.1H NMR(500MHz,DMSO)δ8.27(s,2H),7.86(dd,J=7.9,1.5Hz,1H),7.52-7.45(m,1H),7.33-7.18(m ,2H).13C NMR(126MHz,DMSO)δ160.71,152.00,130.54,124.12,123.50,123.50,121.57,116.45,116.45.ESI-MS m / z:178.0[M+H]+.
Embodiment 2
[0060] Preparation of (E)-4-hydroxyl-3-((2,3-dihydroxybenzylidene)amino)-2-hydrogen-pyran-2-one (1)
[0061]Add 0.1g (0.56mmol) of 3-amino-4-hydroxycoumarin in a single-necked flask, dissolve it with an appropriate amount of absolute ethanol, then add about 0.08g (0.56mmol) of 2,3-dihydroxybenzaldehyde, dropwise After 3 drops of glacial acetic acid, stirred and reacted at room temperature for 5 hours, a yellow precipitate appeared, filtered with suction, washed with absolute ethanol three times, dried, and recrystallized from DMF / acetone (volume ratio: 3:1) to obtain 120 mg of a red solid. , yield 81%; m.p.>250℃, IR(KBr)ν3421,1606,1542,1461,1417,1228,1109,901,754,474cm -1 . 1 H NMR (500MHz, DMSO) δ16.23(s, 1H), 9.85(s, 1H), 7.95(d, J=7.7Hz, 1H), 7.50(t, J=7.6Hz, 1H), 7.24(dd ,J=14.2,7.6Hz,2H), 6.75(d,J=7.8Hz,1H),6.69(d,J=7.4Hz,1H),6.44(t,J=7.7Hz,1H).ESI-MS m / z:295.8[M-H] - ; HRMS (ESI) m / z 296.0562[M-H] - (calcd for 296.0564, C 16 h 10 NO 5 ).
Embodiment 3
[0063] Preparation of (E)-4-hydroxyl-3-((2,4-dihydroxybenzylidene)amino)-2-hydrogen-pyran-2-one (2)
[0064] The preparation process is the same as 1, the raw material salicylaldehyde is replaced by 2,4-dihydroxybenzaldehyde, the yield is 81%; yellow solid, m.p.>250℃, IR(KBr)ν3427,1619,1538,1392,1316,1234,1136, 1031,759,445cm -1 . 1 H NMR (500MHz, DMSO) δ12.79(s, 2H), 10.94(s, 1H), 9.80(s, 1H), 7.97(d, J=7.3Hz, 1H), 7.61(dd, J=18.6, 7.9 Hz,2H),7.40-7.29(m,3H),6.53(s,1H).ESI-MS m / z:295.9[M-H] - ; HRMS (ESI) m / z 296.0562[M-H] - (calcd for 296.0564, C 16 h 10 NO 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com