Preparation method of 1, 6-II-D or 1-<18>O stable isotope labeling glucose
An isotope labeling and glucose technology, which is applied in the chemical industry, can solve the problems of long preparation period and cumbersomeness of stable isotope labeling glucose, and achieve the effects of flexible and diverse preparation conditions, simple purification method and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
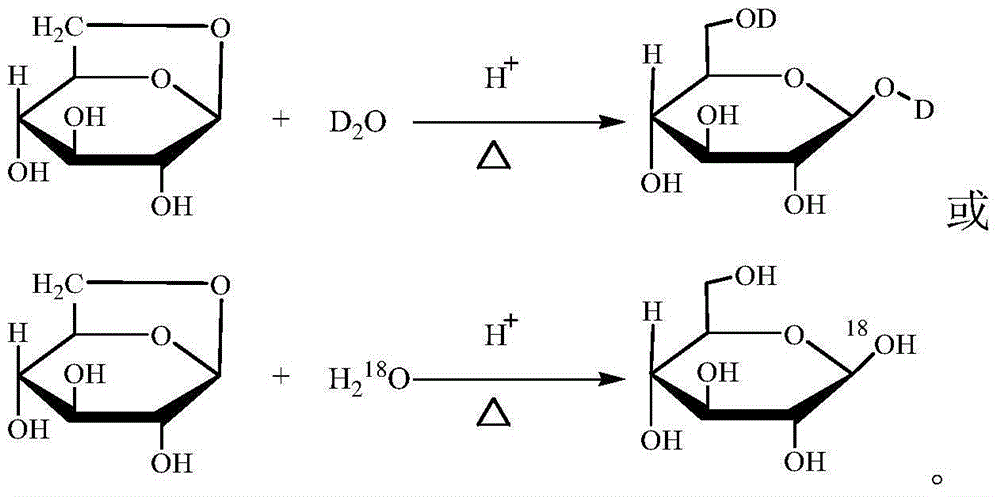
[0017] The present invention provides a 1,6-di-D or 1- 18 O stable isotope labeled glucose (referred to as 1,6-di-D-glucose or 1- 18 The preparation method of O-glucose) belongs to the technical field of biochemical industry. The preparation method mainly uses 1,6-shrink-β-D-glucopyranose (referred to as inner ether sugar) as raw material, and D 2 O or H 2 18 O is a solvent, and in the presence of strong inorganic acid, it is hydrolyzed at 50-200°C to hydrolyze the inner ether sugar to obtain D or 18 Glucose hydrolyzate is labeled with O stable isotope, and then the hydrolyzate is purified to obtain D or 18 O Stable isotope labeling of glucose products. The chemical reaction utilized can be expressed as:
[0018]
Embodiment 1
[0020] Preparation of glucose (abbreviation: 1,6-di-D-glucose) labeled with D stable isotope at the first and sixth hydroxyl H atoms. Including the following steps:
[0021] (1) Raw materials: inner ether sugar, D 2 O;
[0022] (2) Hydrolysis: put the inner ether sugar into the hydrolysis reactor, add 0.01mol / L sulfuric acid containing 0.01mol / L of the inner ether sugar 2 O, the solution was heated to 90°C, reacted for 120 minutes to add a molecule of D stable isotope-labeled water to the inner ether sugar, and then cooled to room temperature to obtain a solution containing 1,6-di-D-glucose;
[0023] (3) Purification of 1,6-di-D-glucose: Add sodium hydroxide solution to the solution obtained in step (2) to make the solution pH=7, distill under reduced pressure to a thick solid, slowly add 8 times the solid A large amount of absolute ethanol, reflux and stir for more than 30 minutes, while hot double-layer quantitative filter paper suction filtration, remove solid sodium sul...
Embodiment 2
[0025] Preparation of 1,6-di-D-glucose. Including the following steps:
[0026] (1) Raw materials: inner ether sugar, D 2 O;
[0027](2) Hydrolysis: put the inner ether sugar into the hydrolysis reactor, add 0.5mol / L hydrochloric acid containing 0.5mol / L hydrochloric acid of 10 times the inner ether sugar quality 2 O, the solution was heated to 50°C, reacted for 60 minutes to add a molecule of D stable isotope-labeled water to the inner ether sugar, and then cooled to room temperature to obtain a solution containing 1,6-di-D-glucose;
[0028] (3) Purification of 1,6-di-D-glucose: Add potassium hydroxide solution to the solution obtained in step (2) to make the solution pH=7, distill under reduced pressure to a thick solid, slowly add 10 times the solid A large amount of absolute ethanol, reflux and stir for more than 30 minutes, while hot double-layer quantitative filter paper suction filtration, remove solid potassium chloride insoluble in ethanol, then collect the filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com